Review Article - Modern Phytomorphology ( 2022) Volume 16, Issue 5
Overview of Obesity and Pharmacological Significance of Sita Ashoka (Saraca asoca) Helpful in management of obesity and other Diseases: A Review
Shivam Vashist1*, Kamal1, Pooja Rathi1, Anish1, Rohit1, Dhirender Kaushik and Shivkant Sharma2M Pharmacy, Department of Pharmaceutical Science, Gurugram University. Postal code 122018, Gurugram, Haryana, India
3M Pharmacy, Department of Pharmaceutical Science, Gurugram University. Postal code 122018, Gurugram, Haryana, India
4M Pharmacy, Department of Pharmaceutical Science, Gurugram University. Postal code 122018, Gurugram, Haryana, India
5M Pharmacy, Department of Pharmaceutical Science, Gurugram University. Postal code 122018, Gurugram, Haryana, India
6Professor, Department of Pharmaceutical Science, Gurugram University. Postal code 122018, Gurugram, Haryana, India
7Assistant Professor, Department of Pharmaceutical Science, Gurugram University. Postal code 122018, Gurugram, Haryana, India
Shivam Vashist, M Pharmacy, Department of Pharmaceutical Science, Gurugram University. Postal code 122018, Gurugram, Haryana, India, Email: shivamvashist123456@gmail.com
Published: 30-Sep-2022
Abstract
In the traditional medical system known as Ayurveda, herbal treatments were routinely employed. The indigenous plant known as Ashoka, or Saraca asoca (Roxb.), is a member of the Legume family's Caesalpinaceae subfamily and has significant cultural importance. All of this plant's parts are thought to have significant pharmacological value, and they have been used particularly to treat a variety of gynaecological problems such menorrhagia, leucorrhea, and abnormal uterine bleeding. According to reports, saraca asoca contains a variety of phytoconstituents, including flavonoids, steroids, glycosides, saponins, tannins, carbohydrates, and proteins. It also reportedly has a number of pharmacological effects, including those that are spasmogenic, oxytocic, uterotonic, anti-bacterial, anti-obesity, anti-menorrhagia, anti-cancer, anti-estrogenic This paper discusses the pharmacological facts on the saraca asoca plant, various reported pharmacological activities, phytoconstituents, socio-ethnobotanical uses, and a brief description of obesity. Knowledge about how to make saraca asoca more obese both physically and aesthetically. Saraca asoca has a high concentration of antioxidants that lower oxidative stress and the prevalence of obesity, making it the ideal option for testing for pharmaceutically related chemicals in its endophytes. It is envisaged that this review will gather all the necessary, desirable, and distinctive facts under one roof and will also offer new guidelines for the researchers and pharmaceutical sector to increase the Pharma worth of this natural product
Keywords
Obesity, Antioxidant, Oxidative stress, Lipase inhibitors, Ashoka, pharmacology, Metabolism disorder
Introduction
India boasts a sizable number of the therapeutic plants that nature has to offer, making it what some have dubbed "the medical paradise of the globe." One of the most fabled and revered trees in India are the Saraca asoca (Roxb) De Wilde (Leguminosae) tree, often known as Asoka or Asoca. It is a moderate evergreen tree with many extending and falling hairless branches that can reach heights of up to 10 meters. The blossoms are fragrant, abundant, thick, and orange or red in colour, while the bark is dark brown to grey or black. The leaves are pinnate and 15–25 cm long with 4-6 pairs of oblongs–lanceolate leaflets (Angulo MA et al 2015). The plant is frequently employed in Ayurveda to relieve pain, enhance complexion, heal stomach and blood diseases, as well as to calm the central nervous system (CNS). The bark is praised for its use in treating leucorrhoea and gynaecological issues such uterine bleeding brought on by fibroids. Asoka is quite effective at treating the signs and symptoms of painful menstruation, premenstrual syndrome, and non-specific white discharges in addition to treating fatigue and overall weakness. There have also been reports of the plant's antidiabetic, antiobesity, oxytocic, anticancer, peptic ulcer, antimicrobial, antibacterial, and antioxidant properties. The bark has been found to include various catechols, sterols, tannins, flavonoids, glycosides, leucopelargonidin, and leucocyanidin, according to chemical analysis. Catechol, epicatechol, leucocyanidin, oleic, linoleic, palmitic, and stearic acids are all present in the seeds and pods. There are several compounds found in the leaves and stems, including quercetin, quercetin-3-OL-rhamnoside, kaempferol 3-O-L-rhamnoside, amyrin, ceryl alcohol, and beta-sitosterol (P. Pradhan et al 2009).
Obesity
The World Health Organization (WHO) defines obesity as an abnormal or excessive deposit of fat in adipose tissue that impairs health (World health organization, 2000). Adults with a body mass index (BMI) more than 30 and abnormal fat distribution are considered obese, according to clinical definitions. This definition, however, falls short since it fails to take into account the difference between subcutaneous and abdominal/visceral fat distribution in the body. An unbalanced distribution of fat has been linked to obesity and associated comorbidities. Obesity is linked to important systemic changes in the body. Increased release of free fatty acids (FFA) from visceral fat depots and metabolic instability, including insulin resistance, are associated with visceral (or abdominal) obesity (Bruckert E 2008). Hypertrophied intra-abdominal adipocytes may undergo hyperlipolysis, increasing FFA flow to a variety of organs, including the liver. A compromised liver could produce more hepatic glucose and become more insulin resistant as a result of increased FFA flow. Reduced apolipoprotein B breakdown and increased production of triglyceride-rich lipoproteins (TG) are both associated with hepatic insulin resistance (Despres JP & Lemieux 2006). Obese individuals experience an influx of macrophages into adipose tissue, which leads to a persistent low-grade form of inflammation. The altered metabolic profile of obese people may also be influenced by proinflammatory chemicals like interleukin (IL)-6 and tumor necrosis factor (TNF-). Table 1 an indication of the inflammatory state associated with visceral obesity is an increase in inflammation markers, such as plasma levels of C-reactive protein (CRP) (Despres JP & Lemieux 2006).
Table 1 Shows the classification of obesity by WHO
| Weight status | BMI Cut-Off Points (kg/m²) |
|---|---|
| Underweight | <18.5 |
| Normal range | 18.5–24.9 |
| Overweight | ≥ 25 |
| Pre-obese | 25.0–29.9 |
| Obese class I | 30.0–34.9 |
| Obese class II | 35.0–39.9 |
| Obese class III (Severely) | ≥ 40 |
Classification
The WHO classification of weight status
As in other guidelines that have adopted the WHO classification, the term ‘pre-obese’ has been replaced by ‘overweight’ (World Health Organization 1998).
Symptoms of Obesity
The study's review of the literature reveals the following symptoms of obesity: diarrhoea, heartburn, acid regurgitation, gastroesophageal reflux disease (GERD), gastro intestinal irritable bowel (IBL) syndrome, nausea, vomiting, early satiety, upper abdominal pain, lower abdominal pain, bloating, post-prandial fullness, hard stools, decreased stools, increased stools, loose watery stools, and post-prandial fullness (G. D. Eslick 2011).
Causes of Obesity
There are several different causes of obesity induced in body are:
1. Physical inactivity
2. Overeating
3. Genetics
4. A diet high in simple carbohydrates
5. Frequency of eating
6. Medications
7. Psychological factors
8. Diseases such as hypothyroidism, insulin resistance, polycystic ovary syndrome, and Cushing's syndrome are also contributors to obesity.
Drug Induced Obesity
Several pharmacologic agents provoke obesity. Ex: Glucocorticosteroid, sulfonylurea (SU), and thiazolidinediones (TZD) (Aronne LJ et al. 2001).
Oxidative Stress and Obesity
It is known that oxidative stress (OS) has a role in pathological processes like obesity, diabetes, cardiovascular disease, and atherogenic processes. ROS occur under physiological settings, in many disorders, and inflict direct or indirect damage to several organs. According to reports, systemic OS may be brought on by obesity and, in turn, OS is linked to abnormal adipokine production, which aids in the development of the metabolic syndrome (Esposito, K et al 2006). According to BMI, the percentage of body fat, LDL oxidation, and TG levels, CRP sensitivity and other oxidative damage biomarkers are higher in obese people; in contrast, antioxidant defence indicators are lower in relation to body fat percentage and central obesity (Chrysohoou, C et al 2007), (Hartwich, J et al 2007). A study revealed that an obese person's OS stress and inflammation are significantly increased by a diet high in fat and carbs (Patel, C et al 2007) Table 2.
Table 2 Obesity Problems Caused By Oxidative Stress
| S. No | Obesity-related diseases |
|---|---|
| 1 | Heart diseases |
| 2 | Oncology problems |
| 3 | Peripheral vascular disease |
| 4 | Liver failure |
| 5 | Psychology problems |
| 6 | Insulin resistance and diabetes |
| 7 | Gout |
| 8 | Obstructive sleep apnoea, |
| 9 | Asthma |
| 10 | Rheumatological and orthopaedics problems |
Pathophysiology of Obesity
Most scientists concur that body weight or adiposity is actively regulated or defended based on observations that individual adult body weight is extremely stable and resistant to short-term experimental up or down perturbations under consistent environmental conditions (Schwartz MW et al 2017). New information supports the idea that obesity is a disease, transferring the responsibility from the individual to the physiology. It also suggests that the elevated body weight/adiposity in many obese patients is defended just as it is in normal weight subjects (Hall KD, & Guo J 2017). With the finding of more than 140 genetic chromosomal areas linked to obesity, results from genome-wide association studies imply a hereditary propensity for fat (Fall T, et al 2017). The central nervous system has a substantially enriched expression of genes associated with BMI and overall obesity (Locke AE et al 2015). Only a small number of genes, nevertheless, have been found to have a significant impact on BMI thus far. These genes, which are paternally expressed along a specific area of chromosome 15, encode elements of leptin and melanocortin signaling as well as those that cause Prader-Willi syndrome (Speakman JR et al 2006). Common obesity is believed to be caused by a multitude of genes with modest effects, as opposed to such monogenetic cases. Most people agree that environment, lifestyle, and genetic predisposition combine to cause obesity. The existence of genes that increase the risk of being obese has been the subject of several theories. According to the "thrifty" gene hypothesis, during the course of human evolution, genes favouring high fuel efficiency and energy intake were chosen over ones promoting energy consumption (Amy M. Heck et al 2000). According to the "drifty" gene concept, when humans first developed tools for using fire and weapons around 2 million years ago, the evolutionary pressure for genes that kept body weight and adiposity to a minimum loosened because they are no longer under danger from predators, a random gene drift results in an increase in adiposity (Deng, Y.& Scherer P.E 2010).
Treatment of Obesity
1. Reducing food intake either by amplifying inhibitory effects of anorexigenic signals or factors (those that suppress food intake) or by blocking orexigenic signals or factors (those that stimulate food intake).
2. Blocking nutrient absorption (especially fat) in the gut.
3. Increasing thermogenesis by uncoupling of fuel metabolism from the generation of ATP, thereby dissipating food energy as heat.
4. Modulating fat or protein metabolism or storage by regulating fat synthesis/lipolysis or adipose differentiation/apoptosis. Enhanced fat or protein turnover might reduce body weight by affecting either food intake or energy expenditure.
5. Modulating the central controller regulating body weight (i) by altering the internal reference value sought by the controller or (ii) by modulating the primary afferent signals regarding fat stores that are analyzed by the controller. This approach would have the potential advantage of forcing the endogenous controller to regulate multiple pathways of energy balance and minimize compensation.
Quick Relief Medication
When combined with a customized low-calorie, low-fat diet and exercise program, orlistat (both prescription and over-thecounter) can aid in weight loss. People with obesity who may also have high blood pressure, diabetes, high cholesterol, or heart disease use orlistat on a prescription. Orlistat is also used to assist people in preventing re-gaining lost weight after weight loss. The drug orlistat belongs to the group of drugs known as lipase inhibitors. It operates by stopping a portion of the ingested fat from being absorbed in the intestines. The body then excretes this unabsorbed fat in the form of faeces (Begum S N et al 2014) Table 3.
Table 3 Medications that may cause weight gain and treatment options
| Drugs Promote Weight Gain | Alternative drugs that may promote weight loss or be weight neutral | |
|---|---|---|
| Psychiatric/neurologic treatments | ||
| 11 | Antipsychotics: olanzapine, clozapine, risperidone | Ziprasidone, quetiapine |
| 22 | Antidepressants: selective serotonin reuptake inhibitors, tricyclic antidepressants | Bupropion, nefazodone |
| 33 | Lithium | |
| 44 | Antiepileptic drugs: valproate, gabapentin, carbamazepine Diabetes treatment | Topiramate, lamotrigine, Zonisamide |
| Diabetes treatments | ||
| 11 | Insulin | Metformin |
| 22 | Sulfonylureas | Acarbose, miglitol |
| 33 | Thiazolidinediones | Orlistat, sibutramine |
| Steroid hormones and miscellaneous agents | ||
| 11 | Hormonal contraceptives | Barrier methods |
| 22 | Corticosteroids | Nonsteroidal anti-inflammatory drugs |
| 33 | Pregestational steroids | |
| 44 | Antihistamines | Decongestants, inhalers |
| 65 | β-Blockers, α-blockers | Angiotensin-converting enzyme inhibitors, Ca2channel blockers |
Mechanism of Action of Lipase Inhibitors
Gastric and pancreatic lipases are reversibly inhibited by orlistat, which is how it works. These lipases are crucial for the breakdown of dietary fat. They function by dissolving triglycerides into free fatty acids and monoglycerides that can be absorbed. The serine residues of lipases' active sites are inactivated by orlistat, which binds covalently to them. Free fatty acids are not absorbed because the inactivation of lipases stops the breakdown of triglycerides. R. Guerciolini, 1997 Local lipase inhibition in the gut is the main effect of orlistat. Orlistat does not require systemic absorption to work. It prevents about 30% of dietary fat from being absorbed at the appropriate dosage.
Saraca Asoca (Plant Profile)
The Saraca asoca. S. indica L., locally known as "Sita Asok," S. declinata (Jack) Miq., popularly known as "Red Saraca," and S. Thaipingensis Cantley ex Prain, locally known as "Yellow Saraca" are three of the three species of Saraca that belong to the Caesalpinaceae family and are found in India (PK Warrier et al 2000).
Synonyms: Sorrowless Tree, Asoka Tree, Ashoka Tree, Gapis, Talan, Saraca Indica, Sita Asoka, Saraca bijuga, Saraca pierreana, Saraca harmandiana, Saraca arborescen (M Ali 2008).
Vernacular names: English: Ashokam, Ashok tree; Assamese: Ahok, Ashok, Ashok-goch; Gujarati: Ashopalava; Hindi: Ashok, Sita Ashok; Kannada: Achenge; Arbi: Mir Krem; Malayalam: Hemapushpam, Ashokam; Marathi: Jasundi; Tamil: Asogam; Sanskrit: Kankeli; Telugu: Ashokapatta.
Habitat: It can be found in India's evergreen woods up to an elevation of 750 metres. India as a whole is home to it. Particularly in the Himalaya, Kerala, Bengal, and the entire south. It can be found in the Khasi, Garo, and Lussi hills in the Himalayas, as well as in the districts of Thrissur, Kollam, and Kannaur in Kerala, as well as Patagiri, Kaikatty, and Pothundi in the Palakkad district (SK Jain 1968).
Botanical description: Saraca asoca a little, 7–10 cm tall evergreen tree is known as a saraca asoca. It happens up to an altitude of 750 metres. The leaves are firmly subcoriaceous, oblong, and 6–12 cm long and parpinnate. The pestistipules are intra-petiolar and entirely joined; the leaves are narrowly lanceolate, cork-like at the base, and have a shot. The bark has a warty surface and is dark brown, grey, or virtually black in colour. Due to the presence of rounded or protruding lenticles, stem bark is rough and irregular. Bark that has been channeled is smooth with lenticles and traversely ridged, and occasionally cracked. A thin, continuous, whitish layer can be observed beneath the cork leaver due to fracture splinting that reveals the surface's striated pattern. Flowers smell good. Flowers are polygamous and apetalous, appearing in short, lateral corymbose, axillary panicles; bracts are tiny, deciduous, and the calyx is petaloid. The seeds are compressed, ellipsoid-shaped, and number 4–8 Table 4.
Table 4 shows use of saraca asoca in Ayurvedic Treatment
| S.no | Dosh (Diseases) | Action or Treatment |
|---|---|---|
| 1 | Grahi | improves digestion and assimilation |
| 2 | Trishanashnam | alleviates excessive thirst |
| 3 | Vedanasthapana | improves digestion and assimilation |
| 4 | Krimighna- | kills all infectious agents |
| 5 | Vishasrajit | useful in toxicities and all blood disease |
| 6 | Asrigdaranashanam | management of excessive bleeding during menstruation |
| 7 | Apachijit | useful in management of inflammation of lymph nodes |
| 8 | Varnya | improves complexion of the body |
| 9 | Dahashamanam | Ashoka alleviates burning sensation |
| 10 | Shothajit | useful in management of all edematous conditions |
Ayurvedic Actions:
Traditional Use: Ksheerapaka of its 6-gm bark powder should be administered in Pradara Roga in ladies. It works well for all kinds of aberrant vaginal discharges. Ksheerapaka is also helpful for dysuria, urinary calculi, uterine inertia, and uterine discomfort. Its bark paste should be administered to the area causing the pain. To prevent gynaecological diseases, Chhattisgarh women boil the bark of Ashoka in cow's milk, add sugar, and ingest it once daily for three days. They then repeat the process three months later. On "Ashok Shasthi day," married Hindu women in India consume the flower buds of Saraca asoca to protect their offspring from sadness and anguish. It is recommended for those with mental illness to bathe in Ashok's shade. Native Americans make a specific herbal mala for mental peace and administer it to patients using Sita Ashok root pieces. Patients are instructed to stuff the ground seeds into the pan (betel vine) and consume it on an empty stomach. The healers generally advise boiling the bark with cow's milk and taking the resulting liquid (after removing the bark). Sugar can be added for flavour. As a preventative measure against gynaecological issues, the healers advise every native female to consume this milk once day for up to three days every three months. The bark is boiled in water and made into a decoction by the healers in cases of menorrhagia. Numerous other plants are added to this decoction. The patients receive this concoction every morning on an empty stomach. The bark is also boiled in milk by several healers. Additionally, the decoction is applied externally to wash. The bark is boiled in milk and water by the healers in the case of Safed Pani (Leucorrhoea). Patients are given the mixture when the water evaporates (Navneet kumar Yadav et al 2015) Table 5.
Table 5 Macro and Microscopic characters of S. asoca .
| S. No | Macro-microscopic characters | Saraca asoca |
|---|---|---|
| 1 | Stem bark | Channelled |
| 2 | Phelloderm and Stone cells | Phelloderm represented by 2-3 continuous bands of stone cells |
| 3 | Distribution pattern of fibres in phloem Region | In a group of 3-24 |
| 4 | Mucilage canals and oil cells | Absent |
| 5 | The broadness of Medullary rays | Uni-biseriate |
General Character
Macro and microscopic characters of saraca asoca:
For the saraca asoca plants under study, a substantial number of measurable macro- and micro-morphological characters— both quantitative and qualitative—were acquired. Prior to doing this investigation, we gave several earlier efforts some thought Table 6.
Table 6 Shows Quantitative and qualitative data of Saraca asoca; Data expressed as mean of 10 replicates ± SD for quantitative data only.
| S. No | Characters | Saraca asoca |
|---|---|---|
| 1 | Colour of flower | Saffron |
| 2 | Colour of leaf | Deep green |
| 3 | Presence of apical appendage | Present |
| 4 | Stomata type | Paracytic |
| 5 | Presence of inflorescence bract | 2 |
| 6 | Colour of floral bract | Green, 2 |
| 7 | Persistent nature of floral bracteoles | Persistent |
| 8 | Colour of Stamen | Blue to violet |
| 9 | Presence of apical hood on anther | Absent |
| 10 | Colour of mature staminal disc | Red |
| 11 | Presence of hair on ovary | Dense |
| 12 | Report of presence of polyembryony | Present |
| 13 | Presence of exine perforation | Absent |
| 14 | Type of exine ornamentation | Macro-regulate |
| 15 | number of leaflets | 5 |
| 16 | Mean length of leaflets cm | 16.13 ± 0.6 |
| 17 | Mean width of leaflets cm | 4.41 ± 0.15 |
| 18 | Length of leaf rachis cm | 22.3 ± 1 |
| 19 | Length of stipule mm | 6.03 ± 0.15 |
| 20 | Mean length of petiole mm | 3.8 ± 0.5 |
| 21 | Average number of veins | 24 |
| 22 | Length of pulvinous base mm | 7.6 ± 0.5 |
| 23 | Angle of leaflet apices | 74.09° ± 9.6° |
| 24 | Angle of leaflet base | 70.17° ± 9.37° |
| 25 | Size of stoma & subsidiary cells μm2 | 4933.71 ± 136.77 |
| 26 | Length of guard cell μm | 42.3 ± 3.4 |
| 27 | Width of guard cell μm | 12.9 ± 1.3 |
| 28 | Ostiolar Length μm | 30.95 ± 2.7 |
| 29 | Porous Area % of stomata | 19.82 ± 2.6 |
| 30 | Ratio of area of epidermal cell and subsidiary cell | 1.02 ± 0.03 |
| 31 | Diameter of the inflorescence cm | 4.2 ± 0.56 |
| 32 | Number of stipules at the base | 2 |
| 33 | Angle between inflorescence axis & stem | 93.79° ± 2.1° |
| 34 | Presence of 3° branch | 0 |
| 35 | Number of flowers per inflorescence | 42 ± 12 |
| 36 | Distance between inflorescence axis and first leaf cm | 5.87 ± 0.7 |
| 37 | Length of 1° inflorescence axis cm | 8.41 ± 0.4 |
| 38 | Distance between two flower cm | 3.2 ± 0.2 |
| 39 | Height of Inflorescence cm | 6.3 ± 0.5 |
| 40 | Total Length of flowers mm | 50.18 ± 1.2 |
| 41 | Breadth of lower floral bracteole mm | 2.32 ± 0.2 |
| 42 | Size of floral bracteole mm2 | 7.9 ± 0.97 |
| 43 | Numbers of hairs on upper part of floral bracteole per 1 mm2 | 37 ± 6 |
| 44 | Numbers of hairs on middle part of floral bracteole | 42 ± 10 |
| 45 | Numbers of hairs on lower part of floral bracteole | 60 ± 4 |
| 46 | Length of hairs on upper part of floral bracteole µm | 106.64 ± 1.9 |
| 47 | Length of hairs on middle part of floral bracteole µm | 60.96 ± 2.1 |
| 48 | Length of hairs on lower part of floral bracteole µm | 112.7 ± 6.2 |
| 49 | Length of floral bract mm | 2.48 ± 0.12 |
| 50 | Breadth of floral bract mm | 2.18 ± 0.04 |
| 51 | Size of floral bract mm2 | 3.97 ± 0.22 |
| 52 | Mean Length of androecium mm | 22.83 ± 1.2 |
| 53 | Number of anthers | 7 |
| 54 | Length of filaments mm | 27.22 ± 1.6 |
| 55 | Area of anther mm2 | 1.03 ± 0.08 |
| 56 | Distance of filament attachment µm | 0.58 ± 0.09 |
| 57 | Number of Hairs on anther (U) µm | 8 ± 1 |
| 58 | Number of Hairs on anther M | 7 ± 1 |
| 59 | Number of Hairs on anther L | 20 ± 2 |
| 60 | Angle of proximal notch anther | 133.20° ± 9.6° |
| 61 | Height of inner edge μm | 30.50 ± 4.1 |
| 62 | Mean length of gynoecium mm | 25.6 ± 2.1 |
| 63 | Mean length of ovary mm | 5.3 ± 0.7 |
| 64 | Mean area of ovary mm2 | 2.59 ± 0.6 |
| 65 | Mean length of style mm | 18.64 ± 1.1 |
| 66 | Mean length of stigma mm | 0.66 ± 0.07 |
| 67 | Mean breadth stigma mm | 0.59 ± 0.07 |
| 68 | Mean length of hair near stigma µm | 746.3 ± 22.7 |
| 69 | Mean number of hairs on ovary | 31 ± 1 |
| 70 | Mean length of hairs on ovary µm | 36.4 ± 2.67 |
| 71 | Mean number of hairs on stalk | 36 ± 2 |
| 72 | Depth of attachment of ovary on disc μm | 746.82 ± 22.7 |
| 73 | Mean length of pods cm | 12.15 ± 2.1 |
| 74 | Mean breadth of pods cm | 2.9 ± 0.6 |
| 75 | Thickness of seeds mm | 27.36 ± 2.13 |
| 76 | Size of cotyledon mm2 | 1345.89 ± 122.62 |
| 77 | Mean breadth of colpa µm | 6.59 ± 0.32 |
| 78 | L/B Ratio of pollen grains | 6.31 ± 0.3 |
| 79 | Height of anulus µm | 1.94 ± 0.2 |
| 80 | Apocolpium index | 12.66 ± 1.4 |
| 81 | Colour of floral bract | Light green |
| 82 | Persistent nature of floral bracts | Deciduous |
Work Reported on Saraca Asoca
Phytochemical Works Reported: The leaves of Saraca asoca were found to include carbohydrates, phenolic compounds, flavonoids, phenols, tannins, saponin, glycosides, and steroids (21).
The bark of the saraca asoca has been shown to include saponin, carbohydrates, phenols, tannins, glycosides, and other chemicals (Sikaris K 2004).
Biological Works Reported
Anti-Microbial activity the study investigated in the anti-microbial properties of Saraca asoca against Candida albicans and Aspergillus Niger, as well as Bacillus sublitis and Pseudomonas aeruginosa, two gramme positive and gramme negative bacterial species. The antifungal medicine fluconazole and the common antibacterial antibiotic ciprofloxacin were compared to the petroleum ether ethanol extract of leaves. Based on the research, it was discovered that ethanol extract had strong antibacterial characteristics.
Anti-Oxidant and Anti-Hyperglycemic activity Sunil Kumar and his colleagues tested Saraca asocas anti-hyperglycemic and antioxidant capabilities in rat models in which diabetes was artificially caused by streptozotocin. Comparative research was done on leaf extracts made from petroleum ether, chloroform, and methanol. It was discovered that oral administration of extracts significantly reduced blood sugar levels. Methanol extract has a significantly higher anti-hyperglycemic capability than the other extracts. All of the extracts shown significant antioxidant activity (P 0.05) at a dosage of 500 g/ml. Studying the anti-hyperglycemic and antioxidant activities in streptozotocin-nicotinamide-induced diabetic rat models revealed that ethanolic extract has a substantial ability to lower blood glucose levels, according to Shanti Bhushan Mishra and Vijayakumar M. The primary oxidation related enzymes dismutase, peroxidase, glutathione, and superoxide were reduced and returned to normal levels by the ethanolic extract.
Anti-Keratinizing activity the anti-proliferative and anti-keratinizing effects of Saraca asoca were investigated in rat uteri that had undergone estradiol-induced keratinizing metaplasia. It was discovered that estradiol-induced thickening of the epithelial layers may be reduced with the use of a methanolic extract of Saraca asoca. Additionally, it decreased serum oestrogen levels, demonstrating its importance as an anti-keratinizing agent (Lastra G et al 2006).
Anti-Pyretic activity the investigation into the antipyretic action of Saraca asoca on Wistar rat models with experimentally produced pyrexia by Brewer's yeast revealed that its seeds are an effective antipyretic agent at a specific dose.
Cardioprotective activity the cardioprotective effects of Saraca asoca were investigated by Viswanatha Swamy et al. in rat models with cyclophosphamide-induced cardiotoxicity. According to the study, an alcoholic extract of the bark might undo the modifications that the drug cyclophosphamide had caused in the rats.
Anti-ulcer activity to test the anti-ulcer activity of Saraca asoca, Maruthappan V et al. performed a study using albino rat models. The two albino rat models employed were stomach ulcers brought on by aspirin and pyloric ligation. It was discovered that the anti-ulcer efficacy of flowers in an aqueous suspension. The ulcer index was decreased by aqueous suspension, indicating that it may be used as an anti-ulcer medication (Lastra G et al 2006).
Anti-Helminthic activity the aqueous extract of Saraca asoca, methanol, and chloroform are all linked to the anti-helminthic activity, according to the study that was mentioned. Pheretimaposthuma adult earthworms were used to test this action. All of these Saraca asoca extracts displayed significantly more potent antihelminthic action than the conventional antihelminthic medications utilised in the study.
Analgesic activity an investigation using albino rat models was done by Angad Verma et al. The tail immersion method and the formalin-induced pain method were used to inflict pain on the models. Petroleum ether, chloroform, methanol, and water extracts were the ones that had their analgesic potency tested. The extract dosages employed were 200 and 400 mg/kg. Methanol extract was discovered to be a more effective analgesic.
Haematoprotective activity According to Chetan Kumar Dubey and his team's study results, Saraca asoca exhibits hemoprotective properties. They tested its action in rat models that had anaemia brought on by phenyl hydrazine. The oral treatment of Saraca asoca for 14 days drastically reduced MCV and WBC levels, increased Hgb and RBC levels, and decreased MCV and WBC levels, which are all indicators of anaemia.
Anti-Arthritic activity Saraca asoca's anti-arthritic properties were investigated by Subramanian Saravanan et al. Female Wistar rats were used in the investigation, which included paw swelling, body weight, levels of lysosomal enzymes, protein-bound carbohydrates, serum cytokines, urine collagen, and joint histology. The plant's methanolic extract was found to have strong antiarthritic properties. The bodyweight increased and the paw thickness decreased after the methanolic extract was given.
Anti-Epileptic activity an investigation into this quality used albino mouse models. Anti-epileptic characteristics were assessed using the maximal electroshock (MES) and pentylenetetrazol (PTZ)-induced seizure models. The plants ethanolic extract demonstrated antiepileptic qualities in both models, supporting its anti-epileptic activity.
Anti-Diarrhoeal activity the anti-diarrheal action of Saraca asoca was examined in an in-vivo investigation by S. Panchawat et al. and Sisodia S. S using albino rat models. Castor oil was used to intentionally produce diarrhoea in albino rats. At a dosage of 200 mg/kg, it was shown that Saraca asoca bark extracts had effective anti-diarrheal effects.
List of Agents and Factors Triggering Obesity
Etiological factors: Obesity, in general, is caused by consuming more energy than one expends; in children, greater consumption of fats and carbohydrates as well as a lack of physical activity has been associated to obesity. The "thrifty gene theory," which postulates that some populations may have genes that determine increased fat storage, the latter when experiencing periods of starvation, thus providing a survival advantage, is the basic theory of the disease's cause. However, in the modern world, an abundance of fat leads to obesity and Type 2 diabetes mellitus (T2DM) (Bravo P et al 2006). However, it has been hypothesised that in the early phases of our evolution, very efficient systems were created to capture the meagre energy available, which resulted in the creation of adipose tissue. Lack of industrial development required lengthy days of strenuous physical labour to produce meagre amounts of food. For subsequent use, this energy would efficiently collect. The number of cases has increased as a result of lifestyle and dietary changes of obese subjects; obesity is thought to play a significant role in a number of health issues. The foetal origins hypothesis of chronic diseases is another idea that explains how obesity develops. This shows that inadequate nutrition during pregnancy and stunted foetal growth are risk factors for developing chronic illnesses that alter the programming of the body's physiology, metabolism, and skeletal structure (Amirkhizi F et al 2007). Obesity can come from a breakdown in several signalling networks in the central nervous system (CNS), which controls appetite, calorie intake, and weight gain (Bravo P et al 2006).
Epidemiology: The world's biggest public health issue, particularly in industrialized nations, is obesity (Chan R.S et al 2010). Obesity increases mortality and the likelihood of developing colon cancer, diabetes, and cardiovascular illnesses (Dulloo A.G et al 2010). A significant body of research has shown that obesity and overweight are substantial contributors to co-morbidities such T2DM, cardiovascular disease, numerous malignancies, and other health issues that can increase morbidity and death. The associated medical expenses are likewise high. Therefore, it is crucial to take a public health approach when creating populationbased measures to prevent excessive weight gain. However, the increased prevalence of obesity has not been effectively addressed by public health intervention efforts. The definition of overweight and obesity, variations related to age and ethnicity, health effects, and factors causing the development of obesity are discussed in this paper. It also presents a critical review of the efficacy of current public health strategies for risk factor reduction and obesity prevention (Sánchez F et al 2005).
Adipose Tissue: According to Dulloo (Fonseca-Alaniz et al 2007), brown adipose tissue, which has multilocular adipocytes and many mitochondria and expresses high levels of uncoupling protein 1 (UCP-1), is responsible for the tissue's thermogenic activity, while white adipose tissue stores fat. We discovered that one of the features of white adipose tissue is the presence of various cell types, including fibroblasts, preadipocytes, mature adipocytes, and macrophages. Depending on whether this tissue is visceral or subcutaneous, it is quite diverse (Steffes, M et al 2006). Due to the hyperplasia and hypertrophy of their adipocytes, white fat deposits significantly rise in obese animals (Fonseca-Alaniz et al 2004). The diapedesis of monocytes to visceral adipose stroma is facilitated by hypertrophic-hyperplastic adipocytes' lower density of insulin receptors and higher beta-3 adrenergic receptor, which starts a proinflammatory cycle between adipo- and monocytes (20). Studies conducted recently have revealed that white adipose tissue plays a producer function for several chemicals with endocrine, paracrine, and autocrine activity in addition to being a tissue that stores triglycerides (TG) (26). Plasminogen activator inhibitor-1 (PAI-1), tumour necrosis factor-alpha (TNF-), resistin, leptin, and adiponectin are some of the bioactive molecules known as adipokines or adipocytokines (Hukshorn, C.J et al 2006). These chemicals contribute to the equilibrium of different physiological processes and are predominantly derived from white adipose tissue.
Metabolic Homeostacis and Adipokines
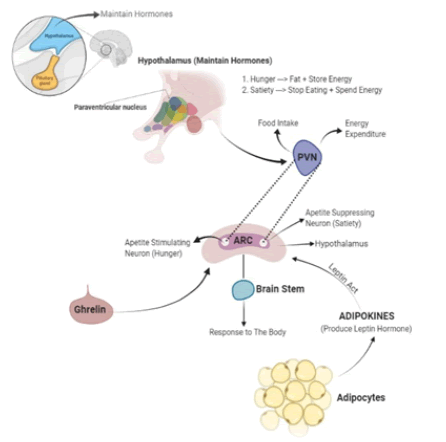
Leptin: In 1994, leptin was found. It is a hormone that is primarily released by adipocytes in direct relation to the nutritional state and the mass of adipose-tissue TG content (27). Leptin circulates in plasma attached to plasma proteins before being secreted by adipose tissue. It enters the CNS by diffusion, capillary binding in the median eminence, and saturable transit across the choroid plexus recipient. The hypothalamus's ventro-medial nucleusLeptin increases the production of melanocyte-stimulating hormone, cytokine receptor kinase 2 (CK2), and cocaine-amphetamine-regulated transcriptor (CART) molecules, which, via paracrine, trigger receptors 3 and 4 of the lateral melanocortin nucleus, resulting in satiety (26, 33). Leptin reduces intracellular lipid levels in skeletal muscle, the liver and pancreatic beta cells by inhibiting lipogenesis and promoting lipolysis. This enhances insulin sensitivity. The locus coeruleus nucleus, which is activated by the limbic system, causes the sympathetic nervous system to be activated, which increases resting energy expenditure by stimulating dopamine reuptake and inhibiting the pleasure of eating (Hukshorn C.J et al 2004). Although it has been suggested that glucose metabolism is the primary determinant of leptin secretion because the concentration of circulating leptin decreases under fasting or caloric restriction conditions and increases in response to food intake, catecholamines influence the production of leptin and other leptin production regulators include the glucocorticoids (26, 31). It has been hypothesised that the apparent reduction in anorexigenic effects and weight loss is the result of a mechanism of resistance to it because obesity is connected with elevated leptin levels (Hukshorn C.J et al 2004). Leptin directly affects macrophages during inflammation, increasing their phagocytic activity. Proinflammatory cytokine production also has an impact on T-cells, monocytes, neutrophils, and endothelial cells. Leptin’s inflammatory action is demonstrated by the fact that it causes C-reactive protein (CRP) levels to rise after administration (Cachofeiro, V et al 2006). The hormone's circulating levels drop with weight loss, which in turn lowers the plasma levels of inflammation-related indicators associated with obesity (Ouedraogo R et al 2007). Leptin increases the proliferation and migration of endothelial cells and smooth muscle cells, enhancing oxidative stress and vascular inflammation in addition to favouring atherosclerosis (English PJ et al 2002). It's important to remember that leptin can also be produced in the placenta, spinal cord, stomach, muscle, and possibly the brain, increasing its regulating function Figure 1.
Figure 1: Role of leptin receptor in our body.
TNF-α (Tumor Necrosis Factor Alpha): One of the earliest cytokines to be discovered, TNF-, is implicated in the systemic inflammatory response and has also been connected to the emergence of insulin resistance, obesity, and diabetes (Steffes, M et al 2014). Its irregular synthesis has a role in the pathophysiology of the obesity-associated metabolic syndrome and is primarily produced by monocytes, lymphocytes, adipose tissue, and muscle. As a result of increasing the release of free fatty acids (FFA) in adipocytes, blocking the synthesis of adiponectin, which has a high concentration of insulin-sensitizing activity in adipose tissue, and interfering with the activity of tyrosine-residue phosphorylation in the first substrate of the insulin receptor, which is required for progression of the intracellular signal of the hormone, TNF- activity on insulin (27). TNF- causes an inflammatory state in adipose tissue, endothelial dysfunction, and finally atherogenesis by activating nuclear factor B (NF— B), which increases the expression of adhesion molecules on the surface of endothelial cells and vascular smooth muscle cells (27).
IL-6 (Interleukin-6): This cytokine has a variety of functions, including those related to defence, inflammation, and tissue damage. It is produced by immune system cells, fibroblasts, endothelial cells, skeletal muscle, macrophages, and adipocytes. BMI, insulin resistance, and sensitivity to carbs are all correlated with circulating levels of IL-6 (27). IL-6 also affects glucose tolerance by negatively regulating visfatin; in addition, it inhibits adiponectin secretion; and in animal models, it increases TG levels by promoting gluconeogenesis and glycogenolysis while decreasing glycogenesis.
Adiponectin: Weight loss is associated with this adipokine's high levels (26). Additionally, adiponectin enhances insulin sensitivity, inhibits key gluconeogenic liver enzymes, slows the release of glucose and muscle from the liver, and accelerates the oxidation of fatty acids and glucose utilisation (27). Adiponectin exhibits strong anti-inflammatory and antiatherogenic properties because it prevents endothelial cell activation, foam cell formation by macrophages, adhesion of monocytes to endothelial cells, and TNF-expression (26). It also lowers CRP levels and boosts the production of nitric oxide (NO) in endothelial cells. When atheromatous plaques form, its globular isoform suppresses cell growth and the generation of ROS brought on by low-density lipoprotein (LDL) oxidase. A lack of adiponectin often causes leukocyte adhesion and a drop in NO in the arterial walls, which leads to persistent vascular inflammation. Finally, it was discovered that adiponectin expression and secretion are strongly inhibited by TNF- and IL-6.
Adipsin: Adipsin is an adipocyte-secreted, relatively tiny serine protease that has a favourable correlation with obesity, insulin resistance, dyslipidemia, and cardiovascular disease. The rate at which fatty acids from Lipoprotein lipase (LPL) are absorbed by adipocytes and subsequently transformed into TG appears to be regulated by adipsin. The molecular underpinnings of the pathophysiology of illnesses associated with obesity are still not fully understood. In addition to being an organ for storing energy, adipose tissue also functions as an endocrine organ. It releases a variety of substances that have endocrine, paracrine, and autocrine effects. Adipose tissue produces biologically active chemicals called adipokines, like adipsin. They contribute to the equilibrium of energy and to the metabolism of glucose and lipids.
Resistin: It has been suggested that the adipokine resistin (RSTN), which is produced by mature adipocytes and macrophages, may represent the link between obesity and insulin resistance (8). The authorized gene symbol for this adipokine is RETN, and it is a member of the family of secreted proteins known as cysteine-rich Found in inflammatory zone (FIZZ). Circulating resistin levels are proportional to the degree of obesity but are unrelated to the degree of insulin resistance; it has hyperglycemic characteristics. Due to it predominate generation of monocytes and its association with IL-6 levels; RSTN is a link to the inflammatory environment.
Gherlin: A strong orexigenic factor, ghrelin is the endogenous agonist of the growth hormone secretagogue receptor (GHS-R). Although the majority of the stomach oxyntic cells are responsible for its production and release, complete gastrectomy only reduces plasma ghrelin by 50–60%. The duodenum, ileum, cecum, and colon all release the remaining circulating ghrelin. Obese patients don't exhibit the rapid postprandial decline in circulating ghrelin, which may contribute to sustained food consumption and obesity. Obese subjects also appear to have abnormal postprandial control of ghrelin (Lunagariya NA et al 2014).
Lipotoxicity: Patients with obesity have adipocytes with lower insulin receptor density and higher beta-3 adrenergic receptor density, which increases lipolysis rate and releases FFA. This has several metabolic effects, including an increase in oxygenderived free radical production, induction of insulin resistance, synergistic action of IL-6 and TNF-, and induction of apoptosis in pancreatic beta cells. When considered as a whole, these metabolic effects result in obesity. Different cell lines are affected anatomically and functionally by lipotoxicity. Two mechanisms—adipose tissue malfunctions and lipotoxicity—explain the proinflammatory state and insulin resistance (IR).
Available Treatment in Health Sector
Abbreviations: OTC, over-the-counter; PCOS, polycystic ovary syndrome.
Lipase Inhibitors
The original lipase inhibitor, lipstatin, is derived from a natural substance. It has a 2,3-trans-disubstituted linear alkyl chain at the -site (C6) and -site (C13) of the compound's -propiolactone ring. It has an N-formyl-L-leucine amino acid that is esterbonded to the -alkyl chain. The table on the right displays Lipstatin's composition along with further details. Orlistat is a semisynthetic substance with a structure resembling lipstatin. Only the saturation of the -alkyl chain, where orlistat is saturated and lipstatin has two double bonds in the side chain, distinguishes them from one another. The table to the right displays Orlistat's structure and additional details. A synthetic lipase inhibitor is cétilistat. It contains a bicyclic benzoxazinone ring rather than a -lactone structure like the majority of lipase inhibitors. Although it is a lipophilic molecule as well, the hydro- and lipophilic side chains are different (Birari RB & Bhutani KK 2007). Table 7 The table on the right displays Cetilistat's structure and additional details. Other lipase inhibitors, such as those derived from various plant compounds, have been identified. Alkaloids, carotenoids, glycosides, polyphenols, polysaccharides, saponins, and terpenoids are a few examples of these. None of these, however, have been therapeutically employed as lipase inhibitors. The lipophilic substances derived from microbial sources are more effective lipase inhibitors (Gras J 2013). Based on their structural characteristics, lipase inhibitors from microbial sources can be separated into two kinds. Lipstatin, Vali lactone, Percyquinnin, Panclicin A–E, Ebelactone A and B, Vibralactone, and Esterastin are examples of compounds with a -lactone ring. (E)-4-amino styryl acetate-polylysine, and caulerpenyne are examples of compounds without a -lactone ring. On the basis of the chemical makeup of triglycerides and other naturally occurring lipase substrates, lipase inhibitors have also been produced synthetically, such as cetilistat. However, certain synthetic lipase inhibitors lack the -lactone ring and have a different structural make-up.
Table 7 Oral agents for potential combination therapy in obesity management
| Name | Medication class | Mode of action for weight management | Side effects/ safety profile concerns | FDA approval status | Combinatorial trials | Typical net weight loss (kg) |
|---|---|---|---|---|---|---|
| Orlistat | Lipase inhibitor | Reducing bowel-based fat absorption | + | Approved for obesity rx, available OTC | with Sibutramine, metformin and phentermine | |
| ~ 2.3 | ||||||
| Sibutramine | B-phenethylamine | Appetite suppressant and increased energy expenditure | ++++ | Pulled from the US market; 2010 | with orlistat | |
| ~ 3.5 | ||||||
| Phentermine | Sympathomimetic | Appetite suppressant | ++ | Approved for short term obesity rx | with orlistat and topiramate | |
| ~ 4 | ||||||
| Diethylpropion | Sympathomimetic | Appetite suppressant | ++ | Approved for short | – | |
| term obesity rx | ~ 3 | |||||
| Benzphetamine | Sympathomimetic | Appetite suppressant | ++ | Approved for short term obesity rx | – | |
| ~ 2.5 | ||||||
| Phendimetrazine | Sympathomimetic | Appetite suppressant | ++ | Approved for short term obesity rx | – | |
| ~ 2.5 | ||||||
| Naltrexone | Partial opioid antagonist | altered satiety perception | ++ | Approved for alcohol dependence | with bupropion | |
| ~ 1.6 | ||||||
| Bupropion | Antidepressant | altered satiety perception | ++ | Approved for smoking cessation | with naltrexone and Zonisamide | |
| ~ 3.2 | ||||||
| Rimonabant | Cannabinoid antagonist | altered satiety perception | +++ | Pulled from the market in Europe; 2007 | – | |
| ~ 6.5 | ||||||
| Topiramate | Antiepileptic | Appetite suppressant altered satiety perception | +++ | Approved for epilepsy and migraine rx | with phentermine and rimonabant | |
| ~ 2.8 | ||||||
| Zonisamide | Antiepileptic | Appetite suppressant Altered satiety perception | +++ | Approved for epilepsy rx | with bupropion | |
| ~ 7.5 | ||||||
| Metformin | Biguanide antidiabetic | Unclear Mild anorexiant | + | Approved for diabetes and PCOS rx | with orlistat | |
| and rimonabant | ~ 2 | |||||
| Lorcaserin | Sympathomimetic | Appetite suppressant; Serotonin 2c agonist | +++ | Approved for monotherapy of obesity | – | |
| ~ 3.7 |
Mechanism of Action of (LI)
Lipstatin and orlistat, two lipase inhibitors, have local effects on the digestive tract. Due to their lipophilicity, they are only little absorbed in the circulation therefore, they have no impact on systemic lipases. They Shows how lipase inhibitors work to prevent the breakdown of fat. These inhibitors create a stable complex by covalently attaching as an ester to the serine hydroxyl group at the active site on pancreatic and stomach lipases. As a result, the enzyme undergoes a conformational shift that exposes the catalytic active site. The hydroxyl group on the serine residue is acylated when the active site is made visible. The enzyme is thereby irreversibly rendered inactive. Triglycerides are `expelled undigested with faeces because the inactive lipase is incapable of hydrolysing fats into absorbable fatty acids and monoglycerides. With this method of action, calorie intake from food fat is constrained, which lowers body weight. Lipase inhibitors' primary function is to suppress lipases in the gastrointestinal system; they have little ``to no effect on proteases, amylases, or other digestive enzymes (42). Although cetilistat lacks the -lactone ring, it has a bicyclic structure. It functions similarly to a standard -lactone-structured lipase inhibitor Table 8 and Table 9.
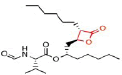
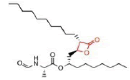
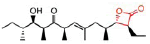
Table 8 Shows that the examples of Lipase inhibitors
| DRUG | ORLISTAT | Valilactone | Panclicin D | Ebelactone | Vibralactone |
|---|---|---|---|---|---|
| STRUCTURE | 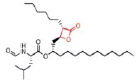 |
 |
 |
 |
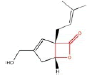 |
| IC-50 VALUE | 1,2 · 10−1µg/ml | 1,4 · 10−4µg/ml | 3,9 · 10−1µg/ml | 1,0 · 10−3µg/ml | 4,0 · 10−1µg/ml |
Table 9 Shows that Orlistat structure, chemical formula and molar mass
| Drug Name | Orlistat |
|---|---|
| IUPAC | [(2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl] tri decan-2-yl]- (2S)-2-formamido -4-methylpentanoate[33] |
| Chemical formula | C29H35NO5[33] |
| Molar mass (g/mol) | 495,7[33] |
Conclusion
The disease of obesity is brought on by dietary imbalance, overeating, and a few neurological conditions. Similar to several hormonal changes, including ghrelin and leptin. Obesity is widespread and ubiquitous everywhere. It can happen to anyone at any age, but around the age of 40, over half of cases start to manifest. Due to fast food, stressful jobs, pollution, and unhealthy lifestyle choices, the likelihood of getting obese has significantly increased in India. India is fortunate to have a rich legacy in the plant world. A number of native plants, including Sarraca asoca and Panax ginseng, have been used in traditional Indian medicine to treat obesity. There are probably a lot of more therapeutic plants with strong antioxidant and phytochemical characteristics. Therefore, there is a lot of need for more research on medicinal herbs to demonstrate the science behind their ability to prevent obesity. This review focuses on the plant saraca asoca (Ashoka), which has a high level of antioxidant properties that lower oxidative stress and is traditionally thought to be helpful in the treatment of obesity. Therefore, saraca asoca has been chosen to conduct a scientific investigation to establish scientific evidence in support of its traditional claim.
References
Angulo MA, Butler MG, Cataletto ME. Prader- Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest 38: 49–63. [Google Scholar] [Cross Ref]
P. Pradhan, L. joseph, V.Gupta,R.Chulet,H.Arya,R.Verma,A.bajpai. Saraca asoca (Ashoka): A Review, School of pharmaceutical sciences, jaipur national university, Jaipur, rajasthan, india, jocpr, 1: 62-71 [Google Scholar] [Cross Ref]
Bruckert E: Abdominal obesity: a health threat (in French). Presse Med; 37: 1407–1414. [Google Scholar] [Cross Ref]
Despres JP, Lemieux I: Abdominal obesity and metabolic syndrome. Nature; 444: 881–887 [Google Scholar] [Cross Ref]
World Health Organization. Report of a WHO consultation on obesity. Obesity: preventing and managing the global epidemic. World Health Organ.: Geneva, [Google Scholar] [Cross Ref]
G. D. Eslick, Obesity Comorbidity Gastrointestinal symptoms and obesity: a meta-analysis, The Whiteley-Martin Research Centre, Discipline of Surgery, The University of Sydney, Nepean Hospital, Penrith, New South Wales, Australia, International Association for the Study of Obesity, (1) [Google Scholar] [Cross Ref]
Aronne LJ. Obesity and weight management, In Nobel J, ed. Textbook of PrimaryCare Medicine, 3rd ed. St. Louis, MO: Mosby; 2001, pp. 485–96. [Google Scholar] [Cross Ref]
Esposito, K.; Ciotola, M.; Giugliano, D. Oxidative stress in the Metabolic Syndrome. J. Endocrinol. Invest., 29, 791–795. [Google Scholar] [Cross Ref]
Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, I.; Papademetriou, L.; Economou, M.; Stefanadis, C. The implication of obesity on total antioxidant capacity apparently healthy men and women: The ATTICA study. Nutr. Metab. Cardiovasc. Dis., 17, 590–597. [Google Scholar] [Cross Ref]
Hartwich, J.; Goralska, J.; Siedlecka, D.; Gruca, A.; Trzos, M.; Dembinska-Kiec, A. Effect of supplementation with vitamin E and C on plasma hsCPR level and cobalt-albumin binding score as markers of plasma oxidative stress in obesity. Genes Nutr., 2, 151-154. [Google Scholar] [Cross Ref]
Patel, C.; Ghanim, H.; Ravishankar, S.; Sia, C.L.; Viswanathan, P.; Mohantym, P.; Dandona, P. Prolonged reactive oxygen species generation and Nuclear Factor- kB activation after a high-fat, high-carbohydrate meal in the obese. J. Clin. Endocrinol. Metab. 92, 4476–4479. [Google Scholar] [Cross Ref]
Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: An Endocrine Society scientific statement. Endocr Rev; 38: 267–96. [Google Scholar] [Cross Ref]
Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology; 152:1718–27. e3. [Google Scholar] [Cross Ref]
Fall T, Mendelson M, Speliotes EK. Recent advances in human genetics and epigenetics of adiposity: pathway to precision medicine. Gastroenterology; 152:1695–706. [Google Scholar] [Cross Ref]
Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature ; 518:197–206. [Google Scholar] [Cross Ref]
Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and alternative perspective: the ’drifty gene’ hypothesis. Int JObes (Lond); 32:1611–7. [Google Scholar] [Cross Ref]
Dr. Amy M. Heck; Dr. Jack A. Yanovski; Dr. Karim Anton Calis (2000). Orlistat, a New Lipase Inhibitor for the Management of Obesity, Pharmacotherapy 20(3), 270–279. [Google Scholar] [Cross Ref]
Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. , 1212, E1–E19. [Google Scholar] [Cross Ref]
Begum S N, Ravikumar K, & Ved D K, (2014). 'Asoka'–an important medicinal plant, its market scenario and conservation measures in India. Current Science, 107(1), 26-28. [Google Scholar] [Cross Ref]
PK Warrier; VPK Nambier; PM Ganpathy. Some important medicinal plants of the western ghats, India: A Profile. Int. Dev. Res. Cent., New Delhi ; 343-360. [Google Scholar] [Cross Ref]
M Ali. Pharmacognosy, CBS Publishers & Distributors, New Delhi. 2008; 668-669 [Google Scholar] [Cross Ref]
SK Jain. Medicinal Plants. National Book Trust, New Delhi, 1968; 124. [Google Scholar] [Cross Ref]
Navneet kumar Yadav, Karan Singh Saini, Zakir Hossain, Ankur Omer, Chetan sharma, jiaur r. gayen, Poonam singh,K.R Arya, and R.K.Singh, SARACA ASOCA bark extract shows in vitro antioxidant, antibreast cancer Activity and does not exhibit toxicological effects, oxidative medicine and cellular longevity,hindawi,2015. [Google Scholar] [Cross Ref]
Sikaris, K. The clinical biochemistry of obesity. Clin. Biochem. Rev. 2004, 25, 165–181. [Google Scholar] [Cross Ref]
Lastra, G.; Manrique, C.M.; Hayden, M.R. The role of beta-cell dysfunction in the cardiometabolic syndrome. J. Cardiometab. Syndr. 2006, 1, 41–46. [Google Scholar] [Cross Ref]
Bravo, P.; Morse, S.; Borne, D.; Aguílar, E.; Reisin, E. Leptin and hypertension in obesity. Vasc. Health Risk Manage. 2006, 2, 163–169. [Google Scholar] [Cross Ref]
Amirkhizi, F.; Siassi, F.; Minaie, S.; Djalali, M.; Rahimi, A.; Chamari, M. Is obesity associated with increased plasma lipid peroxidación and oxidative stress in women. ARYA Atheroscler. J. 2007, 2, 189–192. [Google Scholar] [Cross Ref]
Chan, R.S.; Woo, J. Prevention of overweight and obesity: How effective is the current public health approach. Int. J. Environ. Res. Public Health 2010, 7, 765–783. [Google Scholar] [Cross Ref]
Dulloo, A.G.; Jacquet, J.; Solinas, G.; Montani, J.P.; Schutz, Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int. J. Obes. 2010, 34 (Suppl. 2), S4–S17. [Google Scholar] [Cross Ref]
Sánchez, F.; García, R.; Alarcón, F.; Cruz, M. Adipocinas, tejido adiposo y su relación con células del sistema inmune. Gac. Méd. Méx. 2005, 141, 505–512. [Google Scholar] [Cross Ref]
Fonseca-Alaniz, M.H.; Takada, J.; Alonso-Vale, M.I.; Lima, F.B. Adipose tissue as an endocrine organ: From theory to practice. J. Pediatr. 2007, 83 (Suppl. 5), S192–S203. [Google Scholar] [Cross Ref]
Steffes, M.; Gross, M.; Lee, D.; Schreiner, P.; Jacobs, D. Adiponectin, visceral fat, oxidative stress and early macrovascular disease: The coronary artery risk development in young adults’ study. Obesity 2006, 14, 319–326. [Google Scholar] [Cross Ref]
Hukshorn, C.J.; Lindeman, J.H.; Toet, K.H.; Saris, W.H.; Eilers, P.H.; Westerterp Plantenga, M.S.; Kooistra, T. Leptin and the proin flammatory state associated with human obesity. J.Clin. Endocrinol. Metab. 2004, 89, 1773–1778. [Google Scholar] [Cross Ref]
Cachofeiro, V.; Miana, M.; Martín, B. Obesidad, inflamación y disfunción endotelial. Rev. Esp. Obes. 2006, 4, 195–204. [Google Scholar] [Cross Ref]
Ouedraogo, R.; Gong, Y.; Berzins, B.; Wu, X.; Mahadev, K.; Hough, K.; Chan, L.; Goldstein, B.J.; Scalia, R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Invest. 2007, 117, 1718–1761. [Google Scholar] [Cross Ref]
English PJ, Ghatei MA, Malik IA, Bloom SR, and Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab 87: 2984, 2002. [Google Scholar] [Cross Ref]
Bai T, Zhang D, Lin S, Long Q, Wang Y, Ou H, Kang Q, Deng Z, Liu W, Tao M (December 2014). Operon for biosynthesis of lipstatin, the Beta-lactone inhibitor of human pancreatic lipase. Applied and Environmental Microbiology. 80 (24): 7473–83. [Google Scholar] [Cross Ref]
Lunagariya NA, Patel NK, Jagtap SC, Bhutani KK (2014). Inhibitors of pancreatic lipase: state of the art and clinical perspectives. EXCLI Journal. 13: -897–921. [Google Scholar] [Cross Ref]
Birari RB, Bhutani KK (October 2007). Pancreatic lipase inhibitors from natural-sources: unexplored potential. Drug Discovery Today. 12 (19–20): 879–89. [Google Scholar] [Cross Ref]
Gras J (December 2013). Cetilistat for the treatment of obesity. Drugs of Today. 49 (12): 755–9. [Google Scholar] [Cross Ref]