Research - Modern Phytomorphology ( 2021) Volume 15, Issue 1
Morphological and anatomical study of the genus Sambucus L. (Adoxaceae) in Iran
Elham Amini1, Fatemeh Nasrollahi2, Ali Sattarian1*, Mohsen Isazadeh-Araei1 and Meisam Habibi12Department of Biology, Faculty of Sciences, University of Qom, Qom, Iran
Ali Sattarian, Department of Biology, Faculty of Sciences, Gonbad Kavous University, Gonbad, Iran, Email: Sattarian.ali@gmail.com
Received: 05-Jan-2021 Accepted: 02-Mar-2021 Published: 10-Mar-2021, DOI: 10.5281/zenodo.5078656
Abstract
Sambucus (Adoxaceae) is a tall tree-like shrub distributed throughout temperate and subtropical regions in Europe, Asia, North Africa and North America. Since there is not any morphological and anatomical comprehensive study on Sambucus species in Iran and Sambucus nigra is located in the Arasbaran region with very limited distribution, we have encouraged studying the patterns of morphological variation and anatomical structure among Sambucus taxa.
In this study, the morphological and anatomical structure of 18 populations from two species of Sambucus (S. ebulus and S. nigra) has been considered to evaluate the relationships in this genus. In total, nine quantitative and nine qualitative morphological features were evaluated and measured. Ward’s dendrogram showed two main clusters. The first cluster is composed of a population of S. nigra. The second cluster was composed of two subsets and contained populations of S. ebulus in Golestan, Mazandaran and Gilan provinces. There were two types of epidermal cells, puzzle-shaped in S. ebulus and oblong cells in S. nigra. The stems are rounded shape in both species. The margin is quite wavy in S. ebulus and straight in S. nigra. Both species showed a rounded shape with a wavy margin in petiole cross-sections. Pedicel cross-sections of both species are similar to each other in the general shape of cross-sections and margins. Both are rounded in shape and the margin is quite sinuous shapes in S. ebulus and wavy in S. nigra. The present study showed that a combination of morphological and anatomical data provides reliable evidence for the differentiation of two species of S. nigra and S. ebulus.
Keywords
Adoxaceae, anatomical structure, Iran, morphological, Sambucus.
Introduction
Sambucus L. (Adoxaceae) with 25 extant taxa is a tall tree-like shrub and can be found growing in temperate and subtropical regions in Europe, Asia, North Africa and North America (Mabberley 2008). Sambucus was previously classified in the Caprifoliaceae family but due to new genetic research and reclassification (Donoghue 2003), it now belongs to Adoxaceae family. A large number of variants are known in horticulture (Bean 1951). This genus includes two species in Iran namely, S. ebulus L. and S. nigra L. that distributed in the North, Northwest and Northeast of Iran (Jamzad 1993). In this concept, Sambucus L. is widely known for its species properties, for example, S. nigra L. had its immunomodulatory functions pointed out by Barsett et al. (2012) and S. ebulus L. has anti-inflammatory activity (Ebrahimzadeh et al. 2006). Habitats of Sambucus include places near forests, rivers and as a non-cultivated plant at higher altitudes. It is common in Iran and distributed in moist grasslands or forest margins of Turkey (Kaya et al. 2019). This plant can grow in a vast variety of soil conditions wet or dry, but it can grow in partial shade conditions or under moderate sunlight. Various parts of this plant for example bark, roots, leaves, flowers and fruits have medicinal properties and therefore they are economically used in the food, pharmaceutical and cosmetic industry (Hejný 2001). Sambucus has long been used to treat various illnesses as a diaphoretic, diuretic, astrin¬gent, laxative and emetic (Uncinimanganelli et al. 2005; Merica et al. 2006).
It is a perennial plant characterized by a long, creeping and branched rhizome. Young plants of Sambucus have numerous branches arising from the base. Many of these branches subsequently branch again and form new shoots. It’s characterized by opposite pinnate leaves and the stems terminate in a cluster of flowers. The inflorescences are composed of aromatic white flowers which are clustered in umbrella cymes (JanÄoviÄová 2011). Stems of inflorescences (Pedicels) are generally short, tomentose with two bracteoles (Watson and Dallwitz 2007). The adaxial surface of the leaf is devoid of stomata. Stalk-like extrafloralnectaries reaching up to 10 mm long occur in the nodal regions of the stem between the bases of the leaves and also at the bases of leaflets (Fahn 1987). Stipules are absent on the first two pairs of leaves which develop from hibernated buds in spring (Neubauer 1977).
The morphological and anatom¬ical studies of this genus are not so extensive. SedláÄková et al. (2018) determined the basic morphological characteristics and antioxidant activity of Sambucus nigra L. inflorescences. Alerico et al. (2016) characterized the morphoanatomy and identify the compounds present in the internal part of the stem bark of S. australis through chemical and histochemical methods. Micrographs and descriptions of the anatomy of the root of Sambucus nigra are given by Cutler et al. (1987) and of the stem wood by Metcalfe (1948). Petiole anatomical studies by Amini et al. (2019) show that vascular bundles and the existence of druse crystals are diagnostic characters in species of Sambucus.
Since there is not any morphological and anatomical comprehensive study on Sambucus species in Iran and Sambucus nigra is located in the Arasbaran region with very limited distribution, we have encouraged to study the patterns of morphological variation and anatomical structure among Sambucus taxa. Moreover, due to different populations of Sambucus nigra and S. ebulus collected from different parts of Iran, we expect to resurrect subspecies or verities in these two species and find diagnostic morphological and anatomical characters about this genus.
The specific objectives of this study were as follows: (1) to find morphological characters that could be useful for the diagnosis of taxa; (2) to study the species relationship; (3) to assess the value of anatomical characters in two species of Sambucus in Iran.
Materials and Methods
Morphological methods
In the present study, 18 populations of two Sambucus species were collected from different locations in Iran and preserved in the Gonbad Kavous University Herbarium (GKUH). Identification of populations was carried out based on Flora Iranica(Wendelbo 1965). A list of voucher specimens of the corresponding species is given in Tab. 1.
Table 1. List of the Iranian species of Sambucus used in the present study along with their localities and vouchers preserved in the Gonbad Kavous University Herbarium (Gonbad Kavous, Iran).
| Taxa | Collection data |
|---|---|
| Sambucus nigra L. | Gilan: Arasbaran,Vayghan, Isazade-Araei (803308) |
| S. nigra | Gilan: Arasbaran, Aynalu, Isazade-Araei (803307) |
| S. nigra | Gilan: Arasbaran, Shabkhaneh, Isazade-Araei (803309) |
| S. ebulus L. | Golestan: Gorgan, Tuskestan forest, Isazade-Araei (803315) |
| S. ebulus | Golestan: Golestan National Park, Isazade-Araei (803314) |
| S. ebulus | Golestan: Nowkandeh, Isazade-Araei (803311) |
| S. ebulus | Golestan: Bandar Gaz, Isazade-Araei (803310) |
| S. ebulus | Golestan: Kordkuy, Isazade-Araei (803313) |
| S. ebulus | Mazandaran: Babol, Isazade-Araei (803318) |
| S. ebulus | Mazandaran: Nur, Nurforest, Isazade-Araei (803319) |
| S. ebulus | Mazandaran: Sari, Isazade-Araei (803317) |
| S. ebulus | Mazandaran:6 km to Daryakenar, Isazade-Araei (803326) |
| S. ebulus | Gilan: Lahijan, near to Rasht, Isazade-Araei (803316) |
| S. ebulus | Gilan: Arasbaran, Berenjanestak, Isazade-Araei (803321) |
| S. ebulus | Gilan: Rasht, Isazade-Araei (803322) |
| S. ebulus | Gilan: Lefur, Isazade-Araei (803320) |
| S. ebulus | Gilan: Vayghan, Isazade-Araei (803323) |
| S. ebulus | Gilan: Lahijan, Isazade-Araei (803324) |
Nine quantitative and nine qualitative morphological features were evaluated and measured (Tab. 2). These features were life form, plant height, petiole length, petiole trichome, leaflet number, stipule, leaflet length, leaflet width, pedicel length, pedicel width, anther color, anther trichome, petal color, peduncle length, peduncle width, leaf trichome and leaf length.
Table 2. Quantitative and qualitative morphological characters in Sambucus species.
| No. | Character | No. | Character |
|---|---|---|---|
| 1 | Plant height (mm) | 10 | Life form (shrub 0; herbaceous 1) |
| 2 | Leaf length (mm) | 11 | Anther trichome (glandular 0; linear 1) |
| 3 | Peduncle length (mm) | 12 | Anther color (pale 0; purple 1) |
| 4 | Peduncle width (mm) | 13 | Leaflet number (multi-leaved 0; single-leaved 1) |
| 5 | Leaflet length (mm) | 14 | Petiole trichome (glandular 0; linear 1) |
| 6 | Leaflet width (mm) | 15 | Petal color (yellow 0; purple 1) |
| 7 | Pedicel length (mm) | 16 | Leaf trichome (glandular 0; linear 1) |
| 8 | Pedicel width (mm) | 17 | Stipule (present 0; absent 1) |
| 9 | Petiole length (mm) | 18 | Flower number (less 0; many 1) |
Grouping of the species was obtained by using UPGMA (Unweighted Paired Group Method with Arithmetic mean) and Ward (Minimum spherical cluster method) as well as PCoA (Principal Coordinate Analysis) (Podani 2000). Statistical analysis including ANOVA (Analysis of Variance) and CVA (Canonical Variance Analysis) test was performed to show significant morphological differences. To show variable morphological characters, PCA (Principle Component Analysis) was investigated to identify the most variable morphological characters (Podani 2000). Three times measurements were performed for statistical analysis. These analyses were done by PAST software v.2.17 (Hammer et al. 2012).
Anatomical methods
Fresh leaf materials of the second or third nodes of the stem were fixed in the field with formalin-acetic acid-alcohol (FAA, or removed from herbarium specimens at GKUH. Four cross-sections were measured for each sample to assess the consistency of anatomical characters. All materials were boiled for 15 minutes, then fixed in Carnoy solution (alcohols to acetic acid in proportion 3:1). Materials were kept warm in a tube with previous lotions (the above-mentioned solutions) for 4 hours then the leaves were cleaned. Handmade cross-sections were obtained from petioles, pedicels and stem using commercial razor blades. The cross-sections were obtained using the carmine and methylene green double staining method. Subsequently, the materials were washed in distilled water (1 minute) and dehydrated through ethyl alcohol (70%) and were mounted on microscopic glass slides. Slide sections were studied and photographed.
For leaf epidermal anatomical features, all materials were boiled for 15 minutes, then fixed in Carnoy solution (alcohols to acetic acid in proportion 3:1). The epidermis was separated with H2O2 and acetic acid (1:1), to prepare the leaves. Materials were kept warm in a tube with previous lotions (the above-mentioned solutions) for 4 hours then the leaves were cleaned. After cleaning, the materials were washed in distilled water. Epidermis separation followed. Epidermal samples were stained with 2% aceto-carmine and were mounted on microscopic glass slides. Slide sections were studied and photographed with the help of an Olympus light microscope by using an Olympus DP12 Digital Camera (Japan). Some characters (stomata length/weight, number of stomata, number of epidermal cells) were measured with Image Tools ver. 3.0 and Axio Vision 4.8. (Tab. 3).
Table 3. Leaf epidermal anatomical features of Sambucus.
| Adaxial epidermis | Abaxial epidermis | |||||||
|---|---|---|---|---|---|---|---|---|
| Taxon | Cell shape |
Anticlinal walls |
Stomatal apparatus cell |
Anticlinal walls |
Stomata index (mm2) |
Stomata density (mm2) |
Stomata size (μm) |
Stomata type |
| S. nigra L. | Puz | Wa | - | Irr | 1.92 | 131.70 ± 2.3 | 49.62 × 42.24 | Anemocytic |
| S. nigra | Puz | Wa | - | Irr | 1.67 | 128.50 ± 4.3 | 46.31 × 40.43 | Anemocytic |
| S. nigra | Puz | Wa | - | Irr | 1.59 | 139.32 ± 3.3 | 48.41 × 46.34 | Anemocytic |
| S. ebulus L. | Obl | Str | - | Irr | 3.31 | 180.25 ± 2.1 | 71.32 × 66.24 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.34 | 177.18 ± 1.4 | 65.45 × 53.14 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.43 | 192.23 ± 1.2 | 62.41 × 54.32 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.17 | 181.16 ± 3.2 | 72.43 × 65.30 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.76 | 186.19 ± 2.5 | 65.25 × 51.20 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.32 | 198.14 ± 4.2 | 60.42 × 52.33 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.97 | 175.28 ± 1.7 | 73.40 × 61.30 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.56 | 180.19 ± 3.5 | 65.41 × 55.34 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.11 | 193.14 ± 4.2 | 72.35 × 66.30 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.05 | 199.24 ± 6.5 | 66.40 × 58.34 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.75 | 185.28 ± 1.7 | 72.40 × 64.30 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.88 | 170.19 ± 3.5 | 65.41 × 53.34 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.45 | 193.14 ± 4.2 | 70.35 × 67.30 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.2 | 189.24 ± 6.5 | 69.40 × 59.34 | Anemocytic |
| S. ebulus | Obl | Str | - | Irr | 3.17 | 195.28 ± 1.7 | 71.40 × 62.30 | Anemocytic |
Irr: Irregular; Obl: Oblong; Puz: Puzzle-shaped; Wa: Wavy; Str: Straight.
Some of the most important anatomical characters of epiderms, stems, petioles and pedicels in these species are as follows: transverse section shape, the thickness of collenchyma and sclerenchyma, collenchyma and sclerenchyma layer number, parenchyma thickness, the thickness of phloem and xylem, number of vascular bundles, the thickness of pith, trichome type.
Results
Macro morphological study
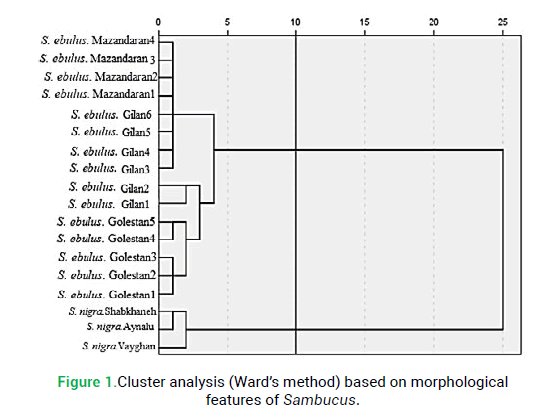
To define the diagnostic value of morphological characters in species delimitations in studied Sambucus species, different clustering methods like UPGMA and Ward produced similar results and both had high cophenetic correlation values (>0.85). Therefore, only the Ward dendrogram is presented here (Fig. 1). The specimens studied in each Sambucus species were placed in species-specific clusters, separating the studied species from each other. Ward’s dendrogram showed two main clusters (Fig. 1). The first cluster is composed of a population of S. nigra. The second cluster was composed of two subsets and contained Golestan, Mazandaran and Gilan populations of S. ebulus.
Figure 1: Cluster analysis (Ward’s method) based on morphological features of Sambucus.
The ANOVA showed significant differences (p<0.05) for the quantitative morphological characters among the Sambucus species (data not given). Moreover, the CVA revealed significant differences among the studied species.
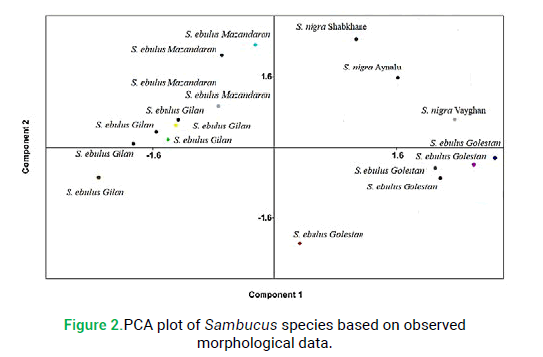
The principal component analysis revealed that two components were providing more than 74% of the total observed variation in studied morphological characters. Studying the component loading was evident that leaflet number, stipule and life form are the most important features in the first factor and plant height, petal color and flower number are most significant in the second factor. These characters may be focused on in the taxonomy of the genus and the delimitation of Sambucus species. PCA ordination based on studied morphological characters separated species into distinct groups (Fig. 2). PCA confirmed the results of cluster analysis by Ward’s method based on qualitative and quantitative features of two studied species (Fig. 2).
Figure 2: PCA plot of Sambucus species based on observed morphological data.
Anatomical study
Selected LM micrographs of cross-sections of epiderms, stems, petioles and pedicels are presented in Fig. 3-Fig. 6. Most characters show significant variability between two species but were constant among different specimens of each species studied.
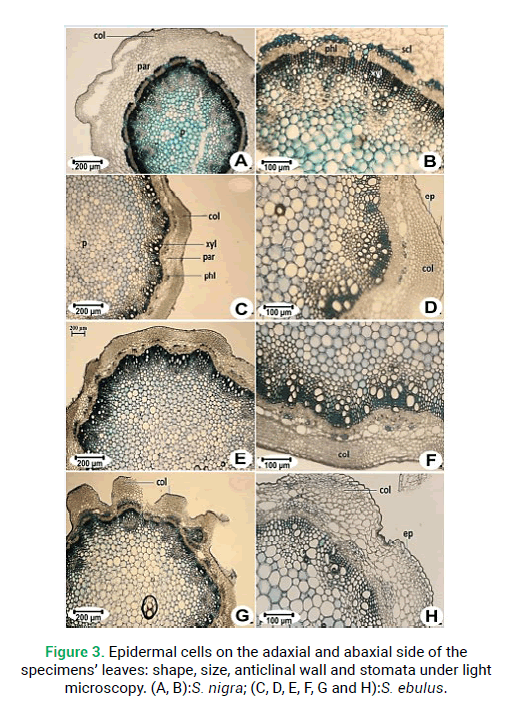
Figure 3: Epidermal cells on the adaxial and abaxial side of the specimens’ leaves: shape, size, anticlinal wall and stomata under light microscopy. (A, B):S. nigra; (C, D, E, F, G and H):S. ebulus.
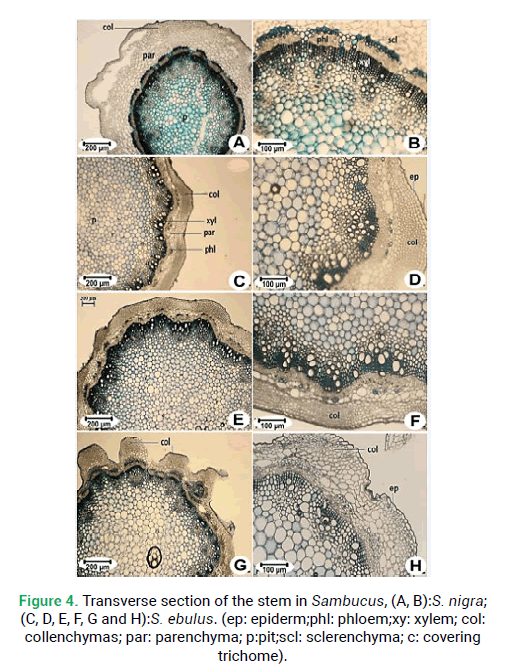
Figure 4: Transverse section of the stem in Sambucus, (A, B):S. nigra; (C, D, E, F, G and H):S. ebulus. (ep: epiderm;phl: phloem;xy: xylem; col: collenchymas; par: parenchyma; p:pit;scl: sclerenchyma; c: covering trichome).
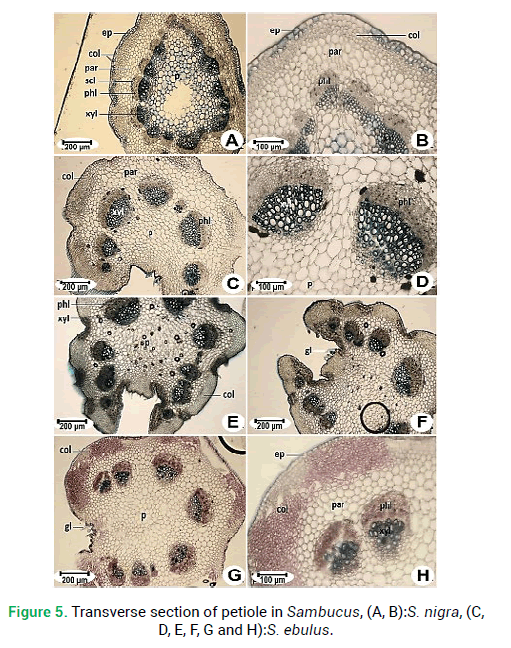
Figure 5: Transverse section of petiole in Sambucus, (A, B):S. nigra, (C, D, E, F, G and H):S. ebulus.
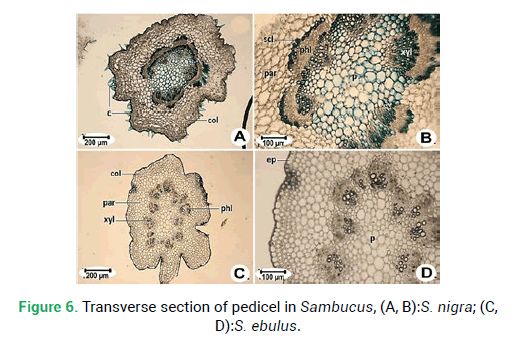
Figure 6: Transverse section of pedicel in Sambucus, (A, B):S. nigra; (C, D):S. ebulus.
Epidermal cell description
Epidermal and stomata characters of the leaves, such as cell shape, anticlinal wall patterns, stomata index, density, size, and types were examined (Tab. 3). There were two types of epidermal cells: Puzzle-shaped and oblong cells could be seen and anticlinal walls have been observed the wavy and sinuous shapes or straight. There are puzzle-shaped cells with wavy anticlinal walls on the adaxial leaf side of S. nigra(Fig. 3A). Oblong cells with straight cell walls were seen in S. ebulus(Fig. 3C, 3E and 3G). Abaxial leaf epidermal cells were irregular, with wavy anticlinal walls in S. nigra(Fig. 5B). All studied species had stomata on the abaxial leaf surface. All treated populations were of the anemocytic stomata type (Fig. 3B and 3D). The smallest in size stomata were observed in S. nigra (Figs. 3B) and the largest were observed in S. ebulus(Fig. 3D). The maximum stomatal index and density were registered in S. ebulus(Tab. 3).
Stem section
Transverse sections from the middle part of the stem revealed the following elements (Fig. 4). The stems are rounded shape in both species. The margin is quite wavy in S. ebulus(Fig. 4G) and straight in S. nigra(Fig. 4A). The epidermis is composed of single-layered cells. The collenchyma tissue is located under the epidermis of the stem. Collenchyma tissue thickness ranging from 279.70 μm in S. ebulus to 154.30 μm in S. nigra. Sclerenchyma tissue thickness in S. nigra is 79.84 μm and that is the maximum sclerenchyma thickness (Fig. 4B). Vascular bundles are arranged in a single circle. The number of vascular bundles is different. There are 16 vascular bundles in S. nigra(Fig. 5A) and 13 in S. ebulus(Fig. 5C). Pith is composed of large orbicular parenchymatous cells with intercellular spaces and pith thickness in S. ebulus (2880 μm) is larger than S. nigra (2596 μm).
Petiole section
Pedicel cross-sections of both species of Sambucus are studied. These two species are similar to each other in the general shape of cross-section and margins. Both are rounded in shape and the margin is sinuous shapes in S. ebulus(Fig. 6C) and wavy in S. nigra(Fig. 6A). The epidermis is composed of single-layered cells. Collenchyma tissue thickness ranging from 140.60 μm in S. ebulus to 168.20 μm in S. nigra.The number of vascular bundles is different. Vascular bundles in S. nigra are 7 (Fig. 6A) and in S. ebulus are 11 (Fig. 6C). Pith thickness in S. ebulus (678.20 μm) is smaller than S. nigra (721.5 μm).
Discussion
Sambucus has little attention in previous morphological and anatomical studies, hence, this study presents the first comprehensive research of this genus in Iran. Species differentiation is the first step toward understanding the evolution of plants and mechanisms of various characteristics and approaches. In our study, morphological and anatomical character data could delimit the studied Sambacus species.
In taxonomy, the use of quantitative morphological characters is traditionally limited to the existence of acondylous diagnostic characters (Amini et al. 2018; Amini et al. 2019). The presence of distinguished combinations of morphological characters supports the existence of two different species, which emphasize previous molecular (Jacobs et al. 2009; Amini et al. 2019). Our morphological investigations on different species of Sambucus confirmed the importance of morphological characters for distinguishing taxa. As it is evident in the PCA graph species are separated by their morphological features. The data on the morphology of Sambucus is relatively poor and only a few works are available. Our results were per PišÅ¥anková (2002), which also found medium-sized, more compact clusters with creamy white medium-sized flowers in the variety Bohatka. In consistence with SedláÄková et al. (2018), our morphological investigations on different species of Sambucus confirmed the importance of the length of inflorescences. Results of the study show that there are some anatomical variations between S. nigra and S. ebulus. However, many features are identical. Our observations are as per previous records in other Dipsacales elements (RuginÄ? and Toma 2007; Gundersen 1910; Petit 1887), While the number of vascular bundles and existence of the crystals of calcium oxalate isa diagnostic character in species studied. Oxalic acid and its salts are usually formed as secondary products of ascorbic and glyoxylic acid metabolism (Williams andWandzilak 1989). They are present in a number of higher plants, including some commonly consumed crop and food plants (Noonan and Savage 1999). Our finding confirms the result of Imenšek et al. (2020) that show Sambucus fruits are a good source of oxalates and interspecific hybridization significantly improves the production and the nutritional quality of fruits.
Our results show that the epidermis is made up of only one cell layer with polygonal cells with thin lateral walls. Stomata have an important role as valuable differentiating characters at different levels of plant taxonomy, ecology, and physiology. Two species of Sambucus show an anemocytic type of stomata. Stomata are present on the abaxial surfaces of leaves only. Habitats and the locality of the species also significantly affect the stomata density. Some scientific reports have shown that genetic and habitat factors and some ecological factors such as water availability, leaf size, stomata size, and density play a significant role for stomata (Blum et al. 1981; Scheettleand Rochelle 2000). S. nigra is a shrub and grows in a dry soil condition, whereas S. ebulus is herbaceous and occupies grasslands or forest margins with high humidity. So high stomatal index and density were observed in S. ebulus and low density in S. nigra. The findings in the current study are in accord with those in other studies relating to the stomata and structure (Miller 1983). The anatomical structure of the stem is in concordance with previous records by Alerico et al. (2016) that the phloem is composed of axial parenchyma cells and radial parenchyma cells, sometimes more elongated than the axial cells, sieve tube elements, companion cells and angle fibers.
As not been studied before Sambucus petiole and pedicel cross-sections, observations could not be compared with other findings. The present study confirmed the leaf anatomical structure by Amini et al. (2019) that vascular bundles and the existence of druse crystals are diagnostic characters. These two species are sympatric species in Iran. These taxa differ in taxonomically important morphological and anatomical characteristics. The genera such as Sambucus seem to have extended their distribution rather recently. So there is a hypothesis that the high similarities in the two species studied can be due to the recent divergence of these species.
In conclusion, the present study provided additional evidence for taxonomists in future studies that they could differentiate two species of S. nigra and S. ebulus by using a combination of morphological and anatomical data.
Acknowledgement
The authors would like to thank Gonbad Kavous University (Gonbad, Iran) and all the persons who helped us in this research work.
References
Alerico G., Vignoli-Silva M.and Lando V. (2016).Morphoanatomical characterization and chemical study of the internal portion of the stem bark of Sambucusaustralis Cham. andSchltdl. Rev Bras Pl Med18: 223-229.https://doi.org/10.1590/1983-084X/15_099
Amini E., Nasrollahi F., Sattarian A., Haji Moradkhani M., Boozarpour S. and Habibi M. (2019).Morphological and anatomical study of the genus Hedera in Iran. Acta Biol. Szeged. 63:91-101. https://doi.org/10.14232/abs.2019.2.91-101
Amini E., Nasrollahi F., Sattarian A., Haji Moradkhani M., Boozarpour S. and Habibi M. (2019).Morphological and anatomical study of the genus Hedera in Iran.Rostaniha20: 144-157.https://dx.doi.org/10.22092/botany.2019.127705.1171
Amini E, Nasrollahi F, Sattarian A, Isazadeh-Araei M, Habibi M (2019) Systematic and molecular biological study of Sambucus L. (Caprifoliaceae) in Iran. Thaiszia J Bot29: 133-150.https://doi.org/10.33542/TJB2019-2-02
Amini E, Zarre Sh. and Assadi M. (2018). Leaf anatomical study of Gypsophila (caryophyllaceae) and allied genera in Iran and its taxonomical implication. Iran. J. Bot. 24: 138-155.https://doi.org/10.22092/ijb.2018.122088.1203
Barsett H., Aslaksen T.H., Gildhyal P., Michaelsen T.E.and Paulsen B.S. (2012).Comparison of carbohydrate structures and immunomodulating properties of extracts from berries and flowers of Sambucus nigra L. Eur J Med Pl2:216-229.http://doi.org/10.9734/EJMP/2012/1335
Bean W.J. (1919). Trees and shrubs hardy in the British Isles (Vol. 2).J. Murray.
Blum A., Gozlan G., and Mayer J. (1981).The Manifestation of dehydration avoidance in wheat breeding germplasm 1.Crop Sci21: 495-499.https://doi.org/10.2135/cropsci1981.0011183X002100040004x
Cutler D.F., Rudall P.J., Gasson P.E.and Gale R.M.O. (1987).Root identification manual of trees and shrubs. A guide to the anatomy of roots of trees and shrubs hardy in Britain and Northern Europe.Chapman and Hall.
Donoghue M.J., Bell, C.D.and Winkworth, R.C. (2003). The evolution of reproductive characters in Dipsacales. Internat J Pl Sci164: S453-S464.https://doi.org/10.1086/376874
Ebrahimzadeh M.A., Mahmoudi M. and Salimi E. (2006).Antiinflammatory activity of Sambucus ebulus hexane extracts. Fitoterapia77: 146-148.https://doi.org/10.1016/j.fitote.2005.11.009
Fahn A. (1987). The extrafloral nectaries of Sambucusnigra. Ann Bot60: 299-308.https://doi.org/10.1093/oxfordjournals.aob.a087448
Gundersen A.L. (1910).Recherchesanatomiquesur les Caprifoliaceae.Thèse, Paris.
Hammer Ø., Harper D.A., and Ryan P.D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica4: 9.http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Hejný S. (2001).Sambucus.In MareÄek, F. et al. Garden Dictionary Teaching 5 R-Ž (ZahradnickýslovníknauÄný 5 R-Ž). Prague, Czech Republic: ÚstavzemÄ?delských a potravináÅ?skýchinformací. p: 174-175. ISBN 80-7271-075-3
Imenšek N., Kristl J., Sem V.and IvanÄiÄ A. (2020). Elderberry (Sambucus spp.) interspecific hybridization and its impact on fruit oxalates.Pl Breeding139: 811-820.https://doi.org/10.1111/pbr.12808
Jacobs B., Lens F., and Smets E. (2009). Evolution of fruit and seed characters in the Diervilla and Lonicera clades (Caprifoliaceae, Dipsacales). Ann Bot 104: 253-276.https://doi.org/10.1093/aob/mcp131
Jamzad Z. (1993).Sambucus L.-In: Assadi, M., Khatamsaz, M. &Maassoumi, A. A. (ed.), Flora of Iran 8. Dipsacaceae-Tehran: Research Institute of Forests and Rangelands.
Kaya Y., Haji E.K., Arvas Y.E., and Aksoy H.M. (2019).Sambucusebulus L.: Past, present and future. In AIP Conference Proceedings.AIP Publishing LLC2155: 020030.https://doi.org/10.1063/1.5125534
Mabberley D.J. (2008).The Plant Book, a Portable Dictionary of Higher Plants. Cambridge University Press, Cambridge.https://doi.org/10.1017/9781316335581
Merica E., Lungu M., Balan I.and Matei M. (2006). Study on the chemical composition of Sambucus nigra L., essential oil and extracts. NutraCos5: 25-27.https://eurekamag.com/research/004/477/004477657.php
Metcalfe C.R. (1948). The elder tree (Sambucus nigra L.) as a source of pith, pegwood and charcoal, with some notes on the structure of the wood.Kew Bulletin3: 163-171.https://doi.org/10.2307/4119753
Miller E.C. (1983).Plant Physiology.Mc Grow-Hill Book Company Inc. New York. U.S.A.
Neubauer H.F. (1977). Morphologischebeobachtungenansaemlingen von Sambucus nigra.18:57-69.https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL7810099342
Nguyá»?n H.V.H.and Savage G.P. (2013).Oxalate content of New Zealand grown and imported fruits.J Food Composition Analysis31: 180-184.https://doi.org/10.1016/j.jfca.2013.06.001
Petit L. (1887). The petiole of dicotyledons from the point of view of comparative anatomy and taxonomy. G. Gounouilhou.38: 13.
Podani, J. (2000). Introduction to the exploration of multivariate biological data.Backhuys Publishers.
RuginaR., and Toma C. (2007).Histo-anatomical aspects of some Lonicera L. species. AnaleleStiintifice ale Universitatii" Al. I. Cuza" din Iasi 53: 26.http://www.bio.uaic.ro/publicatii/anale_vegetala/issue/2007/03-2007.pdf
SchoettleA.W., and Rochelle S.G. (2000).Morphological variation of Pinusflexilis (Pinaceae), a bird dispersed pine, across a range of elevations. Am J Bot 87: 1797-1806.https://doi.org/10.2307/2656832
SedláÄková V.H., Grygorieva O., Fatrcová-Šramková K., Vergun O., Vinogradova Y., Ivanišová E., and Brindza, J. (2018).The morphological and antioxidant characteristics of inflorescences within wild-growing genotypes of elderberry (Sambucus nigra L.). Potravinarstvo 12: 444-453.
Uncinimanganelli R.E., Zaccaro L., and Tomei P.E. (2005). Antiviral activity in-vitro of Urticadioica L., Parietariadiffusa and Sambucus nigra L. J Ethnopharmacol98: 323-327.
Watson L.,and Dallwitz M.J. (1992).The families of flowering plants: descriptions, illustrations, identification, and information retrieval.
Wendelbo P. (1965).Caprifoliaceae in Rechinger, KH (ed.): Flora Iranica, no. 10. Graz: AkademischeDruck-und Verlagsanstalt, 159.
Williams H.E., and Wandzilak T. R. (1989). Oxalate synthesis, transport and the hyperoxaluric syndromes.J Urol 141: 742-747.https://doi.org/10.1016/S0022-5347(17)40999-2