Research Article - Modern Phytomorphology ( 2020) Volume 14, Issue 1
Identifying species and hybrids in the genus juglans by biochemical profiling of bark
F. Likhanov*, R. I. Burda, S. N. Koniakin and M. S. KozyrF. Likhanov, Institute for Evolutionary Ecology, National Academy of Sciences of Ukraine, 37, Lebedeva Str., Kyiv 03143, Ukraine, Email: likhanov.bio@gmail.com
Received: 30-Nov-2019 Accepted: 23-Dec-2019 Published: 02-Jan-2020, DOI: 10.5281/zenodo.4453881
Abstract
The biochemical profiling of flavonoids in the bark of winter shoots was conducted with the purpose of ecological management of implicit environmental threats of invasions of the species of the genus Juglans and their hybrids under naturalization. Six species of Juglans, introduced into forests and parks of Kyiv, were studied, namely, J. ailantifolia Carrière, J. cinerea L., J. mandshurica Maxim., J. nigra L., J. regia L., and J. subcordiformis Dode, cultivar J. regia var. maxima DC. ′Dessert′ and four probable hybrids (♀J. subcordiformis × ♂J. ailantifolia; ♀J. nigra × ♂J. mandshurica; ♀J. cinerea × ♂J. regia and ♀J. regia × ♂J. mandshurica). Due to the targeted introduction of different duration, the invasive species are at the beginning stage of forming their populations, sometimes amounting to naturalization. The species-wise specificity of introduced representatives of different ages (from one-year-old seedlings to generative trees), belonging to the genus Juglans, was determined. J. regia and J. nigra are the richest in the content of secondary metabolites; J. cinerea and J. mandshurica have a medium level, and J. ailantifolia and J. subcordiformis-a low level. On the contrary, the representatives of J. ailantifolia and J. subcordiformis are the most diverse in the content of secondary metabolites. Hybrid forms are notable for the decreased content of flavonoids, which may have a considerable impact on their individual resistance and ecological flexibility. The latter circumstance should be considered in the ecological management of walnut plantings in Kyiv.
The results of biochemical profiling of secondary metabolites from winter shoot bark demonstrated that six investigated species belonged to four sections: Juglans, Cardiocaryon, Rhysocaryon, and Trachycaryon. The hybrids were usually grouped by the sections, represented by the paternal component in the hybrid pair. However, recent results of complete genome sequencing were used by the scientists to prove a relevant evolutionary role of the phenomenon of hybridization in the genus Juglans between the species, separated in the modern areas between the American and Eurasian continents, in particular, the ones, introduced into the Kyiv plantings. The revealed genomic mosaicism forms a new impression about phylogenetic relations within the genus.
The possibility of using the biochemical profiling of winter shoot bark for some utilitarian needs (including the determination of the content of useful biologically active substances and detecting the advantages of hybrids by their performance features) is discussed
Keywords
Juglans, species, hybrid, section, flavonoids, chemosystematics
Introduction
Modern invasions of woody species are the reason for increasing global eco-anxiety. At present, 13,168 species or 3.9% of global flora are naturalized species with secondary areas (van Kleunen et al., 2015). This volume is close to the number of the flora list of the whole of Europe-12,517 natural species.
As of 2013, the global database of invasive trees and shrubs contained 751 species (434 trees and 317 shrubs) from 90 families. 134 alien woody species are known in Europe (Rejmánek & Richardson, 2013). The woody species, which have been or are getting naturalized in the spontaneous flora of Ukraine, are approximately over 180. Among these, 20 are invasive, including 12 transformer species, 29 potentially invasive and 22 completely naturalized species, which have not demonstrated any features of invasion though. 111 remaining species with registered spontaneous seed dispersal are on different stages of naturalization (Burda & Koniakin, 2019). Some alien species of plants are based on hybridization cases prior to or after the introduction. Hybridization cases generate additional genetic diversity, which sometimes enhances the invasive potential of introduction at a specific impact of natural selection. During the formation and distribution of local populations, some may accumulate new diversity via recombination of permanent genetic diversity, especially via the mixture (intraspecific inbreeding between historically isolated populations) or hybridization (spontaneous crossbreeding between different species). For instance, among 20 cases of woody species invasion, revealed by J. F. Gaskin (2017), the hybridization in global flora occurred in 10 cases, while 5 hybrids had a natural local species, as one of the components of the hybrid combination, which generally leads to biodiversity decrease, impoverishment and “homogenization” of the gene fund. There is noted absence or lack of knowledge about hybrid invasive trees due to their morphological latency (crypticity) which limits our possibilities of controlling the invasion in general.
Comparative (referent) methods of identifying the genotypes of the species and hybrids of, vascular plants or any other organisms were used to determine the molecular markers. The markers, based on the polymorphism of proteins (biochemical) or DNA-systems (molecular-genetic markers), are used to solve practical tasks of genetics and the practice of plant selection. A detailed description of the past and the future of genetic improvement of Juglans regia L. specifies the use of SSRmarkers regarding the representatives of the genus Juglans, starting from 2002. It began with the study on 30 microsatellites of J. nigra L. (Bernard et al., 2018). In 2006, this method was applied to determine the low genetic diversity of the local J. nigra in broad-leaved forests of the USA. SSR-markers were used to characterize two species-J. nigra, J. regia and natural hybrids between them- Juglans × intermedia Carr. Later, in 2008, the identification of parental species of this hybrid combination, conducted in the mixed Italian population, confirmed the transfer of J. nigra SSR to the genome of J. regia (Pollegioni et al., 2008). A similar method was applied to identify parental pairs, which may produce hybrid progeny with high survival rates and better wood quality more easily (Bernard et al., 2018).
Molecular methods are currently used to study the genus complex of Juglans L. in several directions: phylogeny and biogeography (Manos et al., 2001; Stone et al., 2009); identification of pure genetic material of vulnerable natural species for their further restoration in natural habitats, preservation of genetic resources via prevention of genomic invasion of local species via the hybridization with alien invasive species (Ross-Davis et al., 2008); identification of hybrid forms of alien invasive species, the consequences of whose occurrence present unexpected threats for local biotic diversity.
At the same time, the information, thus obtained, does not allow studying the level of realization of morpho-physiological features in new habitats, which is especially relevant for ecological research. In addition, the genotype, realized in ontogenesis in terms of new habitats, is the most significant in the aspect of the invasive potential of the introduced specimen. Therefore, the methods of genomics should be supplemented by transcriptomic analysis, proteomics, and metabolomics with the purposes of ecological management.
M. Wink and P. G. Waterman (Wink & Waterman, 1999) highlighted that the study of secondary metabolites, spread in all Angiospermae, on several levels, gave a lot of information about problematic evolutionary, ecological, systematic processes and strategies even at that time. However, the complicated “reticular” nature of the very metabolic manifestations restrained the application of secondary metabolites as taxonomic markers. The authors of the compilation “Chemical Systematic” (Waterman & Gray, 1987) considered it possible to use cyclic polyketides-naphthoquinones, in particular, juglone, as taxonomic markers in the genera Carya Nutt., Juglans L., Pterocarya Kunth (Juglandaceae). However, the substances of the same group were found to be present in the species from other genera-Plumbaginaceae and Droseraceae-plumbagin and Ebenaceae-diospyrin.
The biochemical study of walnuts is underway with utilitarian purposes. Following the development of biochemical methods and technologies, there are new communications, expanding the notions about the composition and properties of secondary metabolites in the representatives of the genus Juglans. For instance, in J. regia, the main group of polyphenols is hydrolyzable tannins. For example, the studies on Juglans in terms of metabolomics, in particular, biosynthesis of nonstructural polyphenols, which could play some role in plant resistance to disease agents, were conducted using J. regia (Bernard et al., 2018). According to the communications in the scientific literature, juglone was identified in five cultivars of J. regia, but they differed in their content and composition of the detected substances. There is a discussion on the possibility of using leaves and pericarps of J. regia as easily accessible sources of both juglone and natural antioxidants. It is noted that after the abscission of leaves and pericarps the phenolic compounds with allelopathic effect get into the soil (Cosmulesku et al., 2011).
During many years of investigations, conducted by Japanese scientists on the East-Asian species of J. ailantifolia Carrière (according to the authors J. mandshurica Maxim. var. sieboldiana Makino), the spectral and chemical data were used to isolate three phenolic glycoside syringates from the bark of the shoots and to describe their structure; one of these glycosides was new (Machida et al., 2009). New substances-juglanperylenone A and juglanperylenone B-were detected in the bark of J. mandshurica shoots among 10 previously indicated by the authors: 4 anthraquinones, 2 coumarins and 4 triterpenoids (Lin et al., 2011; Lin et al., 2013). Considering the relevance of these findings for medicine, the chemotaxonomic significance of new secondary metabolites for Juglandaceae representatives is discussed. 32 components (triterpenes, naphthoquinones, phenolic acids, flavonoids, tannins) were isolated from green pericarps of J. regia. One pentacyclic triterpane and flavonoid were first described for J. regia, and 10 substances were first specified for Juglandaceae in general (Tsasi et al., 2016).
Therefore, the relevance of early immediate identification of natural hybrids among cultivated and naturalized representatives of different Juglans species is determined by the needs of ecological management (determination of implicit environmental threats for natural biotic diversity); solving some disputable problems of systematics and utilitarian requirements (in particular, determining the advantages of hybrids by their performance features). At present we lack the evidence, proving that genetic recombination and heterosis of hybrids enhance their invasive potential, though the accidental occurrence of new hybrid admixtures leads to higher levels of genetic diversity. Obviously, it acquires the status of a common phenomenon, which is still somewhat ignored.
The aim of the work is to identify the species and hybrids of the genus Juglans, cultivated and getting naturalized in forests and parks of Kyiv, using biochemical profiling of secondary metabolites in the bark of winter shoots.
Materials and Methods
Six species of the genus Juglans (Juglandaceae DC. ex Perleb.) were studied: J. ailantifolia Carrière, J. cinerea L., J. mandshurica Maxim., J. nigra L., J. regia L., and J. subcordiformis Dode, cultivar J. regia var. maxima DC. Dessert along with four probable hybrid forms (♀ J. subcordiformis × ♂ J. ailantifolia; ♀ J. nigra × ♂ J. mandshurica; ♀ J. cinerea × ♂ J. regia and ♀ J. regia × ♂ J. mandshurica). All these objects were preliminarily determined by the morphological features.
A sampling of plant material
The experiment samples were selected in Kyiv: the Botanical Garden of the National University of Life and Environmental Sciences of Ukraine, the arboretum of O.V. Fomin Botanical garden of the Taras Shevchenko National University of Kyiv and Feofania park. The experiment samples, their origin, conditions of growth and development were previously described (Burda & Koniakin, 2018) along with the situation with interspecific hybridization in the genus in Ukrainian plantings (Koniakin & Burda, 2019). The bark tissues of winter shoots of the varieties, probable hybrids and the species of the genus Juglans were selected for biochemical profiling. They were collected prior to bud opening in the period from April 17 to April 24, 2018. 54 bark samples were used in the experiment (Tab. 1). Phytochemical studies. The extraction of medium and low-polarity substances from the bark of winter shoots was conducted with 100% methanol (v/v-1/10) for 24 h. The extract was centrifuged for 5 min at 3,000 rpm. The extract, purified from plant residues, was kept at minus 20°.
| Object | Ontogenetic condition of plants | |||
|---|---|---|---|---|
| Tree | Medium and high polewood | Seedling | Total | |
| Species | ||||
| Juglans ailantifolia | 4 | 0 | 0 | 4 |
| J. cinerea | 4 | 0 | 0 | 4 |
| J. mandshurica | 6 | 0 | 0 | 6 |
| J. nigra | 2 | 3 | 0 | 5 |
| J. regia | 8 | 3 | 3 | 14 |
| J. subcordiformis | 6 | 3 | 3 | 12 |
| Hybrids | 6 | 0 | 3 | 9 |
| Total | 36 | 9 | 9 | 54 |
Table 1 : Ontogenetic condition of the plants, from which the bark samples were obtained for biochemical analyses, 2018.
The quantitative content of flavonoids in the methanol extracts of bark was determined by the spectrophotometry method (SP Optizn Pop, South Korea) at the wavelength =419 nm. 300 μl of the extract was successively introduced 200 μl of 0.1 M aluminum chloride solution (AlCl3) and 300 μl of 1 sodium acetate (C3ONa). The calibration curve was built by quercetin (Sigma, Germany). The phytochemical studies were conducted in five repeats (n=5).
The biochemical profiling of the bark of winter shoots was conducted by the HPTLC method using Silicagel G60 plates (Merck, Germany), in the following system: chloroform, acetic acid, methanol, water 60:32:12:8 (v/v/v/v). The distance between the front of the solvent system and the lower edge of the plate was 65 mm (run time-40 min). The plate was dried for 3 min. The individual substances were detected by processing the plate with 1% NP in ethyl acetate (using a natural reagentdiphenylboryloxyethylamine) and 5% polyethyleneglycol (PEG 400) in methylene chloride with subsequent heating for 3 min at 105°. The analysis of chromatograms was conducted in UV at =366 nm with subsequent data processing by photodensitometry method using Sorbfil TLC Videodensitometer (RF).
The obtained results were presented as a minimum (min), maximum (max) values and mean ± standard error (x ± SE). The data and cluster analysis, the principal component analysis was conducted by Statistica 7 (StatSoft Inc., USA, 2004). The significance of the differences between the values (p<0.05) was determined by the analysis of variance (ANOVA) method in the XLSTAT (Addinsoft Inc., USA, 2010).
Results and Discussion
The chromatographic analysis of methanol extracts of the bark revealed 30 phenolic components. The most complicated and changeable biochemical profiles were determined in the bark of J. subcordiformis (up to 20), J. mandshurica (18) and J. cinerea (16). Only 11 phenolic substances were determined in winter shoots of J. nigra. The plants of the latter species were characterized by relatively stable biochemical profiles (Likhanov et al., 2018). Six flavonoids, markers for J. nigra, J. regia and their hybrids, were detected among the determined ones.
In terms of the content of flavonoids, the investigated species of Juglans formed three groups: with a low, medium and high content of these secondary metabolites. The first group included the species J. subcordiformis and J. ailantifolia, the second one-J. cinerea and J. mandshurica, and the third group-J. regia and J. nigra (Tab. 2). At the same time, it is important to consider the degree of variability for the content of flavonoids in the bark, which may be significantly different for plants even within one species. For instance, high variability was notable for plants of J. regia, J. subcordiformis and J. ailantifolia.
| Object | Content of flavonoids, mg/g of dry weight |
СV,% | ||
|---|---|---|---|---|
| Species | min | max | x ± SE | |
| J. subcordiformis а | 1.5 | 3.3 | 2.5 ± 0.35 | 31.0 |
| J. ailantifolia b | 2.1 | 4.4 | 3.3 ± 0.45 | 30.5 |
| J. mandshurica | 2.5 | 4.3 | 3.4 ± 0.37 | 24.8 |
| J. cinerea | 4.1 | 5.6 | 4.9 ± 0.26 | 11.7 |
| J. regia c | 3.9 | 10.3 | 7.1 ± 1.34 | 42.2 |
| J. nigra d | 12.0 | 12.7 | 12.4 ± 0.14 | 2.5 |
| Hybrid | ||||
| J. cinerea× J. regia e | 1.5 | 4.1 | 2.8 ± 0.53 | 42.7 |
| J. nigra× J. mandshurica | 2.2 | 2.5 | 2.4 ± 0.05 | 4.8 |
| J. regia×J. mandshurica f | 2.0 | 2.4 | 2.3 ± 0.08 | 7.5 |
| J. subcordiformis× J. ailantifolia | 3.3 | 3.4 | 3.4 ± 0.02 | 1.6 |
Table 2 : The content of flavonoids in the bark of Juglans species and hybrids (n=5, x ± SE).
For two latter species with a high coefficient of index variability, the difference of flavonoid content between the plants was 1.8-2.3 mg/g, whereas the representatives of J. regia had rather considerable difference (6.4 mg/g). On the contrary, the representatives of J. nigra, accumulating the highest content of flavonoids among the studied species, had rather a low variability coefficient of this index. Flavonoids are known as highly active phenolic substances. They are involved in the regulation of transporting phytohormones, in particular, auxins. They are also notable for high antioxidant potential, inherent bactericide, fungicide, insecticide, and antiviral activity.
is important that their qualitative and quantitative content in live tissues has a considerable impact on the general adaptive possibilities of the plant organism. Therefore, it is reasonable to consider the alleles of chalcone synthase, which is the key in the synthesis of flavonoids, while conducting the analysis of intraspecific genetic polymorphism of J. regia plants. The informative value of this feature is confirmed by the fact that in hybrids, one of the paternal forms of which is highly flavonoid species (J. regia or J. nigra), the content of flavonoids decrease in the range from 2.8-fold to 5.1-fold. The average content of flavonoids in hybrids fluctuates in the range from 2.3 to 3.4 mg/g. High variability of quantitative indices was established for the hybrid J. cinerea × J. regia. On the contrary, the content of flavonoids was consistently low for hybrids J. regia × J. mandshurica and J. nigra × J. mandshurica. According to the literature data, the latter has rather a diverse composition of other phenolic compounds with high biological activity. For instance, the plant tissues of J. mandshurica were found to contain naphthoquinones, naphthalene glycosides, tetralones, flavonoids, diarylheptanoid, galloyl glycosides.
The analysis of biochemical profiles of the investigated species and hybrids of Juglans by the PCA demonstrated that in terms of the qualitative composition of secondary metabolites, phenolic compounds mainly, they form three groups (Fig. 1).
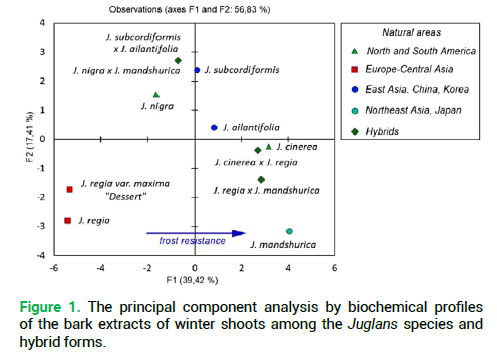
Figure 1. The principal component analysis by biochemical profiles of the bark extracts of winter shoots among the Juglans species and hybrid forms.
The first one includes J. nigra and its hybrid with J. mandshurica. The second one covers J. regia and its large-fruited variety. The third group was formed by J. mandshurica, J. cinerea and their hybrid pairs with J. regia. By the first principal component (39.42%) the species J. ailantifolia and J. subcordiformis are rather close and their contribution to the total dispersion is insignificant. It should be noted that by the feature of resistance to low temperatures the investigated species were rather clearly distributed by the F1 axis (Fig. 1, indicated with an asterisk). For instance, by numerical values of the F1 component, the biggest difference was noted between frost-resistant-J. mandshurica and non-resistant-J. regia. The current natural area of the North American species J. cinerea, which is the most resistant to low temperatures, is somewhat farther north compared to the area of J. nigra (Fig. 2) which is also in agreement with the relevant indices of the principal components (Fig. 1).
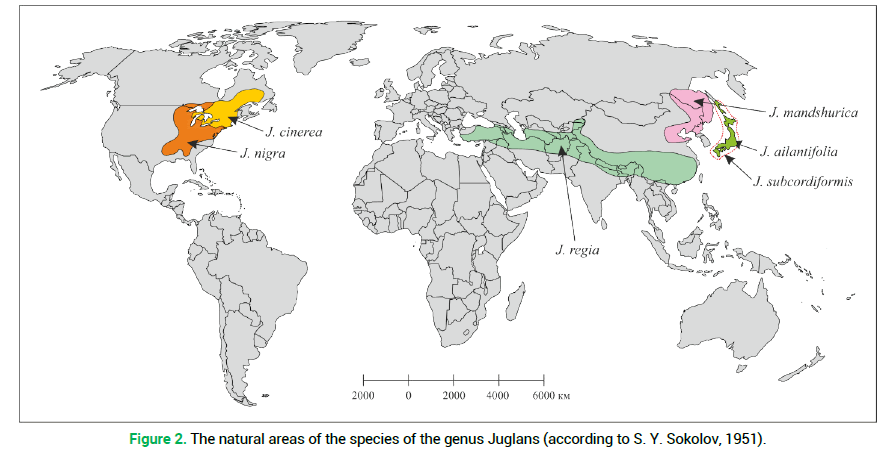
Figure 2. The natural areas of the species of the genus Juglans (according to S. Y. Sokolov, 1951).
Thus, the result of the principal component analysis confirmed the high informative value of the obtained biochemical profiles and their relevance in determining the nature of hybrid forms, including the early stages of ontogenesis. The latter circumstance gives grounds for expectations of early determination of hybrid forms among the seedling growth of the Juglans species under naturalization, but at the stage of seedlings and low, medium and high polewood the latter is almost not possible to taxonomic differentiate.
The cluster analysis by the qualitative composition of phenolic compounds in the bark of winter shoots of six Juglans species demonstrated that the latter belongs to four sections: Juglans, Cardiocaryon, Rhysocaryon and Trachycaryon (Fig. 3).
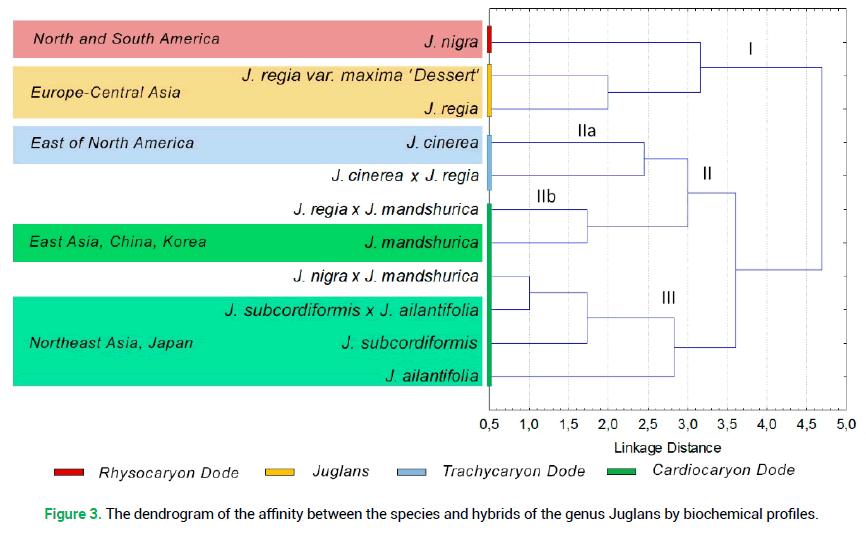
Figure 3. The dendrogram of the affinity between the species and hybrids of the genus Juglans by biochemical profiles.
As a rule, the hybrids, investigated by us, were closer to the sections, represented by the paternal component of the hybrid pair. The exception is found in the hybrid J. cinerea × J. regia. The system of interrelations in the cluster of the Cardiocaryon system (III) was found to be the least definite (Fig. 3). This fact confirms the close and rather mixed biochemical composition of the bark in the species and hybrids of this section.
On the contrary, the species from the North American area of the Rhysocaryon (J. nigra) section and the species with the Eurasian area of the Juglans (J. regia) section are rather close in terms of the presence of flavonoids in the bark of winter shoots (Fig. 3, cluster).
The section structure of the genus Juglans was not completely defined in terms of morphological and molecular-biological notions for a long time. The classification of Juglans on the level of sections remained problematic. The phylogenetic position of the North American species J. cinerea with its restricted area seemed to be especially disputable. The species, involved in our study, are referred to three sections: 1-Juglans (J. regia), 2-Cardiocaryon Dode (J. ailantifolia, J. subcordiformis, J. mandsurica, and J. cinerea), 3-Rhysocaryon Dode (J. nigra) (Konechnaya, 2012); or J. cinerea is isolated into a separate section 4-Trachycaryon Dode (Manning, 1978; APG, 2016). The molecular taxonomy of Juglans almost coincided with the morphological one, except for J. cinerea. The analysis of sequencing chloroplast DNA non-coding spacer (NCS) sequences in seventeen taxons from four sections of Juglans and two species of other genera of Juglandaceae- Pterocarya stenoptera DC. and Carya illinoinensis (Wangenh.) C. Koch did not add any clarity regarding the taxonomic position of J. cinerea (Aradhya et al., 2007). As a result, three monophyletic clades, corresponding to sections Juglans, Cardiocaryon and Rhysocaryon, were detected both in NCS and in combined analyses. The analysis of the plastid genome demonstrated similar results, as it allowed determining three taxonomic branches (Dong et al., 2017). In this case, the American J. cinerea was referred to as “insufficiently determined” species, “closely related” to Rhysocaryon. At the same time, the combined data of the sequences of two nuclear DNA regions demonstrated that the plants of J. cinerea are closer to J. mandshurica and J. cinerea and should be included in the section Cardiocaryon.
The latter results of the complete genome sequencing demonstrated that the evolution of J. cinerea was conditioned by massive introgression of Persian walnut into the genome of J. nigra (Zhang et al., 2019). It explains the presence of the nuclear genome of the Persian walnut and the chloroplast genome of Black walnut in J. cinerea. The cytonuclear irregularity of this type is well-known, remarkable for plants and is explained by the so-called chloroplast capture (Folk et al., 2017).
From the standpoint of J. cinerea ecology, it is also important that the predominant part of its nuclear genome comes from the Asian species (Zhang et al., 2019). In particular, it explains the results of the cluster analysis, conducted by us according to the data of biochemical profiling (Fig. 3). The representatives of the J. cinerea and J. mandshurica species and hybrids are also the closest in terms of the affinity of the qualitative composition of secondary metabolites of the bark. Thus, the results of the analysis of nuclear genomic and biochemical polymorphism are in good agreement.
The results of the complete genome sequencing of the nuclear DNA demonstrated that the species J. regia is also of hybrid origin (Zhang et al., 2019). J. regia plants are capable of easy hybridizing with the representatives of other species of the genus Juglans, which demonstrates the absence of evident reproductive barriers between the relatives. The genetic history of Paradox Hybrids of Juglans is well-known (Suo et al., 2012). Dendrologists and selection breeders paid attention to the inclination of the species of the genus Juglans, introduced to Europe, to have spontaneous interspecific hybridization (Sokolov, 1951; Lypa, 1952; Shchepot’ev et al., 1987). However, these hybrid processes are evidently long-standing in the evolution of history. The morphological and phenological barriers in interspecific hybridization between, for instance, modern J. regia and J. nigra, are described in fine detail (Pollegioni et al., 2012).
Therefore, genomic mosaicism forms a new notion about the importance of hybridization processes in terms of the origin, the evolution of the species of the genus Juglans and their phylogenetic relations. High specificity and informative value of the qualitative composition of secondary metabolites of the one-year-old shoot bark is of interest from the standpoint of determining the role of specific biochemical components and their complexes in implementing adaptive reactions, resistance to negative factors and forming ecological valency of the genus Juglans plants. These features are relevant for predicting the viability and invasive potential of new hybrid forms, spontaneously occurring during the naturalization of the introduced species, in particular, the genus complex of Juglans.
Conclusion
The biochemical profiling of winter shoots was found to be a reliable method of differentiating spontaneous populations of the species of the genus Juglans. Conservative and variable components were detected in the biochemical profiles of the species and hybrids.
The North American and Eurasian species of the sections Rhysocaryon and Juglans are close in the availability of flavonoids. In terms of the content of flavonoids in the bark of winter shoots, the species are divided into the groups with a high level of flavonoids (J. nigra and J. regia), a medium level (J. cinerea and J. mandshurica) and a low level (J. ailantifolia and J. subcordiformis) of them. All the hybrid forms without exceptions were characterized by a decreased content of flavonoids, which may have a significant impact on the individual resistance and ecological flexibility of plants, reducing their invasive potential.
The cluster analysis following the results of biochemical profiling of secondary metabolites in the bark of winter shoots demonstrated their specificity and allowed dividing the investigated species into four sections: Juglans, Cardiocaryon, Rhysocaryonm, and Trachycaryon. As a rule, the hybrids were grouped by the sections, represented by the paternal component in the hybrid pair.
It was determined that the section Cardiocaryon is less structured in the system of cluster interrelations in terms of variability of the composition of phenolic compounds of the bark and thus requires the search for more specific biochemical markers.
Acknowledgments
This study is supported by Bartın University (BAP), Project number: 2019-Fen-B-005.d.
References
- The Angiosperm Phylogeny Group. 2016. APGAn update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. BotJ Linn Soc. 181: 1-20. https://doi.org/10.1111/boj.12385
- Aradhya M.K., Potter D., Gao F., Simon C.J. 2007. Molecular phylogeny of Juglans (Juglandaceae): a biogeographic perspective. Tree Genet Genomes.3: 363-378. https://doi.org/10.1007/s11295-006-0078-5
- Bernard A., Lheureux F., Dirlewanger E. 2018. Walnut: Past and future of genetic improvement. Tree Genet Genomes. 14: 1. https://doi.org/10.1007/s11295-017-1214-0
- Burda R.I., Koniakin S.N. 2018. Spontaneous dispersion of species of the genus Juglans L. in the forests and parks of Kyiv. Russ J biоl Invasions. 9: 95-107. https://doi.org/10.1134/S2075111718020029
- Burda R.I., Koniakin S.N. 2019. The non-native woody species of the flora of Ukraine: Introduction, naturalization and invasion. Biosystems diversity. 27: 276-290. https://doi.org/10.15421/011937
- Cosmulesku S., Trandafir I., Achim G., Baci A. (2011). Juglone contents in leaf and green husk of five Walnut (Juglans regia L.) cultivars. Not Bot Hort Agrobot Cluj. 39: 237-240. https://doi.org/10.15835/nbha3915728
- Dong W., Xu Ch., Li W., Xie X., Lu Y., Liu Y., Jin X., Suo Z. 2017. Phylogenetic resolution in Juglans based on complete chloroplast genomes and nuclear DNA sequences. Front Plant Sci. 8: 1-13. https://doi.org/10.3389/fpls.2017.01148
- Folk R.A, Mandel J.R., Freudenstein J.V. 2017. Ancestral gene flow and parallel organelles genome capture result in extreme phylogenomic discord in a lineage of angiosperms. Syst Biol. 66: 320-337. https://doi.org/10.1093/sysbio/syw083
- Gaskin J.F. 2016. The role of hybridization in facilitating tree invasion. AoB PLANTS. 9: plw079. https://dx.doi.org/10.1093%2Faobpla%2Fplw079
- Konechnaya G.Y. 2012. Family Juglandaceae A. Rich ex Kunth, in Konspekt flory Vostochnoi Evropy (Synopsis of the Flora of Eastern Europe). Tsvelev, N. N. and Gel’tman, D. V. Eds. KMK, St. Petersburg. 1: 190-192 (in Russian).
- Koniakin S.M., Burda R.I. 2019. Imovirnyi ryzyk vynyknennia i nekontrolovanoho vselennia spontannykh hybridnykh form Juglans v Ukraini [The probable risk of rise and non-control disperse of spontaneous hybrid forms of Juglans in Ukraine] In: XIІ Int. Conf. "Synanthropization of the vegetation cover of Ukraine: III All-Ukrainian Scientific Conference, Kyiv, 26-27th of September 2019". Book of scientific articles. Nash format, Kyiv. 85-89 (in Ukrainian).
- Likhanov A.F., Burda R.I., Koniakin S.N. 2018. Biochemical profiling of shoot bark for identifying species and hybrids in the genus Juglans. In: XIІ Int. Conf. "Synanthropization of Flora and Vegetation". Book of Abstracts. (Uzhhorod-Berehove, 20-22 Sept. 2018). AUTHOR-SHARK Press, Uzhhorod, 41.
- Lin H., Zhang Y-W., Bao Y.L., Wu Y., Sun L.G., Yu CL., Huang YX., Wang E.B., Li Y.X. 2013. Secondary metabolites from the stem bark of Juglans mandshurica. Biochem Systemat Ecol. 51: 184-188. https://doi.org/10.1016/j.bse.2013.08.010
- Lin H., Zhang Y.W., Zheng L.H., Meng X.Y., Bao Y.L., Wu Y., Yu C.L., Huang Y.X., Li Y.X. 2011. Anthracene and anthraquinone derivatives from the stem bark of Juglans mandshurica Maxim. Helvet Chim Acta. 94: 1488-1495. https://doi.org/10.1002/hlca.201000462
- Lypa A.L. 1952. Dendrological resources of the Ukrainian SSR and their use. In: Ozelenenie naselennykh mest (Landscaping of Populated Places), Akad. Arkhitekt. Ukr. SSR, Kyiv. 333-345 (in Russian).
- Machida K., Yogiashi Y., Matsuda S., Suzuki A., Kikuchi M. 2009. A new phenolic glycoside syringate from the bark of Juglans mandshurica Maxim. var. sieboldiana Makino. J Nat Med. 63: 220-222. https://doi.org/10.1007/s11418-009-0312-1
- Manning W.E. 1978. The classification within the Juglandaceae. Ann Mo Bot Gard. 65: 1058-1087. https://doi.org/10.2307/2398782
- Manos P.S., Stone D.E. 2001. Phylogeny and systematics of the Juglandaceae. Ann Mo Bot Gard. 88: 231-269.
- Pollegioni P., Olimpieri I., Woeste K., De Simoni D., Gras M., Malvolti M.E. 2012. Barriers to interspecific hybridization between Juglans nigra L. and J. regia L. species. Tree Genet Genomes. 9: 291-305. https://doi.org/10.1007/s11295-012-0555-y
- Pollegioni P., Woeste K., Major A., Scarascia, Mugnozza G., Malvolti M.E. 2009. Characterization of Juglans nigra (L.), Juglans regia (L.) and Juglans x intermedia (Carr.) by SSR markers: A case study in Italy. Sil Gen. 58: 68-78. https://doi.org/10.1515/sg-2009-0009
- Rejmánek M., Richardson D.M. 2013. Trees and shrubs as invasive alien species-2013 update of the global database. Divers Distrib. 19: 1093-1094. https://doi.org/10.1111/ddi.12075
- Ross-Davis A, Huang Z., McKenna J., Ostry M., Woeste K. 2008. Morphological and molecular methods to identify butternut (Juglans cinerea) and butternut hybrids: Relevance to butternut conservation. Tree Physiol. 28: 1127-1133. https://doi.org/10.1093/treephys/28.7.1127
- Shchepot’ev F.L., Pavlenko F.A., Rikhter O.A. 1987. Horikhy (Nut Plants), Urozhai, Kyiv (in Ukrainian).
- Sokolov S.Y. 1951. Family 7, Juglandaceae Lindl. In: Derev’ya i kustarniki SSSR (Trees and Shrubs of the USSR), Akad. Nauk SSSR, Leningrad. 2: 221-255 (in Russian).
- Stone D.E., Oh S.H., Tripp E.A., Rios G.L.E., Manos P.S. 2009. Natural history, distribution, phylogenetic relationships, and conservation of Central American Black Walnuts (Juglans sect. Rhysocaryon). J Torry Bot Soc. 136: 1-25. https://doi.org/10.3159/08-RA-036R.1
- Suo Z., Pei D., Ma Q., Jin X. 2012. Genetic formation of paradox hybrids (Juglans L.) revealed by nrDNA IGS8-ETS1 region. AASRI Procera. 1: 156-165. https://doi.org/10.1016/j.aasri.2012.06.025
- Tsasi G., Milošević-Ifantis T., Skaltsa H. 2016. Phytochemical study of Juglans regia L. pericarps from Greece with a chemotaxonomic approach. Chem Biodivers. 13: 1636-1640. https://doi:10.1002/cbdv.201600067
- van Kleunen M., Dawson W., Essl F., Pergl J., Winter M., Weber E., Kreft H., Weigelt P., Kartesz J., Nishino M., Antonova L.A., Barcelona J.F., Cabezas F.J.,Cardenas D., Cardenas-Toro J., Castano N., Chacon E., Chatelain C., Ebel A.L., Figueiredo E., Fuentes N., Groom Q.J., Henderson L., Inderjit Kupriyanov A., Masciadri S., Meerman J., Morozova O., Moser D., Nickrent D.L., Patzelt A., Pelser P.B., Baptiste M.P., Poopath M., Schulze M., Seebens H., Shu W.S., Thomas J., Velayos M., Wieringa J.J., Pyšek P. 2015. Global exchange and accumulation of non-native plants. Nature. 525: 100-103. https://dx.doi.org/10.1038/nture14910
- Waterman P.G., Gray A.I. 1987. Chemical systematic. Nat Prod Rep. 4: 175-203. https://doi.org/10.1039/NP9870400175
- Wink M., Waterman P.G. 1999. Chemotaxonomy in relation to molecular phylogeny of plants. Ann Plants Rev. 2: 300-341. https://doi.org/10.1002/9781119312994.apr0017
- Zhang B.W., Xu L.L., Li N., Yan P.Ch., Jiang X.H., Woeste K.E., Lin K., Renner S.S., Zhang D.Y., Bai W.N. 2019. Phylogenomics Reveals an Ancient Hybrid Origin of the Persian Walnut. Mol Biol Evol. 1: 11. https://doi:10.1093/molbev/msz112