Research Article - Modern Phytomorphology ( 2020) Volume 14, Issue 1
Features of the in vitro propagation of Pseudotsuga menziesii (Mirb.) Franco
Yaroshchuk R1*, Lisoviy M2, Guz M2, Kovalenko I1 and Zherdetska S12National Forestry and Wood-Technology University of Ukraine, 103 Gen. Chuprynky Street, 79057, Lviv, Ukraine
Yaroshchuk R, Sumy National Agrarian University, 160 H. Kondratieva Street, Sumy, 40021, Ukraine, Tel: 38 0972979385, Email: jaroschukr@ukr.net
Received: 05-Nov-2019 Accepted: 23-Dec-2019 Published: 01-Jan-2020, DOI: 10.5281/zenodo.4449920
Abstract
The analysis of the works of the scientists engaged in the Pseudotsuga menziesii propagation under the in vitro condition has been made. The findings of the authors’ researches on the propagation of the studied species by micro-cloning are presented. The optimal age of the Pseudotsuga menziesii maternal plants to procure initial plant material has been clarified. The explant decontamination (sterilization) scheme has been deduced from experiments, the nutrient medium composition for the in vitro initiation, multiplication and rooting of regenerated plants, as well as the substrate for the adaptation of the obtained clones to soil conditions, have been selected. The obtained results have shown the prospects of the studied species propagation by the proposed method.
Keywords
Adaptation, decontamination, explant, initiation, multiplication, rooting
Introduction
Modern propagation methods, one of which is in vitro, should be used to obtain the genetically valuable planting material of high quality. However, high costs and a total lack of equipment for effective propagation by this method significantly reduce the expected outcome percentage. In this case, the significance of the studied species propagation by this method involves a lack of influence of the periodicity of seed years; the possibility of plant propagation regardless of the time of year; the ability to choose in vitro planting material which has the characteristics being of interest to researchers; the obtaining of planting material that is genetically identical to maternal plant; the in vitro propagation using a small area; the propagation of explants without taking them out from the juvenile phase, etc (Dunstan et al. 1992, Ramage et al. 2002, Pullman et al. 2009).
The Pseudotsuga menziesii in vitro propagation and the successful adaptation of explants to soil conditions are mentioned only in the few scientific works. The sterilization of explants of the studied species is known to be of great importance for successful micro-cloning, on which any precise data are missing in the literature. In particular, the percentage of the explants that have been successfully adapted to the open ground conditions after their decontamination is not clearly specified. The successful outcomes of the in vitro propagation have been mentioned in the works of (Gupta et al. 1985). The bursting of apical and axillary buds of trees of the studied species has been achieved by these authors on the newly formed basal medium (DCR) without growth regulators. The bud elongation was observed on 1/2 of the DCR concentration with 0.3% activated charcoal (DCR-1). Axillary buds redeveloped after sub-culturing when shoots were cultivated on DCR with 0.2 mg/l-1 of BAP. These buds were repassage in DCR-1 medium. Subculturing of 7-10 shoots of Pseudotsuga menziesii during the year may result in obtaining more than 100 clones from one explant when using the above medium.
A significant contribution has been made by (Winton et al. 1977), while studying the Pseudotsuga menziesii embryo, being placed in the nutrient medium, containing vitamin B1, inositol, sucrose and 0.05 or 0.1 mgl/-1 benzyl amino purine (BAP).
A significant contribution to the methods of sterilization and propagation of Pseudotsuga menziesii by micro-cloning has been made by (Khalil et al. 1959), who recommend that the isolated explants be provided with a little amount oxygen and a source of energy in the form of sucrose, glucose or fructose, as well as mineral elements. Clones grew well in the nutrient medium under white fluorescent light. The organic substances influencing favorably the explant development included urea.
Abdoulaye et al. (2005) state that for more effective micro cloning of introduced plant it is necessary to pass through the five stages of sterilization (to immerse in 20% bleach (containing sodium chloride) for an hour, then in 5% solution of sodium chloride for an hour, to rinse in distilled water and hold it over a flame for 3 seconds, and one more time for 5 seconds in 20 seconds). After successful sterilization and beginning of micro cloning, the fastest growth rate of the introduced plant was observed with the low concentrations of BAP (from 0 to 0.045 μmol•L-1), while the high concentrations of BAP (from 0.448 up to 4.527 μmol•L-1) caused their growth inhibition.
Summing up the literature data (Douglas 1914, Durzan et al.1987, Gupta et al.1987 b, Konnert et al. 2006), we can conclude that since the beginning of the 19th century Pseudotsuga menziesii has proved itself to be the promising species for the creation of highly productive, biologically sustainable, artificial forest and decorative plantings of various types and purposes on the territory of Europe, where its plantations are grown on the area of over 800 thousand hectares. The long-standing practical experience in creating the artificial forest plantings of an exotic plant indicates that this species is promising for further use during the creation of forest plantations. The preliminary analysis of the literature on the study of the biological, ecological, silvicultural and economic characteristics of the introduced plant enables to assume that the Western Forest-Steppe of Ukraine is a favorable region for the cultivation of this species in forest and landscape plantings (Guz et al. 2011, Guz et al. 2012, Yaroshcuk et al. 2013). However, it should be mentioned that there is a limited amount of information on the effective method of reproduction of selective valuable genotypes of the studied species (Morris et al. 1990, Ritchie et al. 1992, Ritchie et al. 1997).
The goal of the study is to determine the efficient method of the in vitro propagation of Pseudotsuga menziesii.
The goal provides for the fulfillment of the following objectives:
1) To determine the most efficient method for explant decontamination
2) To identify the optimum age of plants, from which it is expedient to procure explants
3) To choose the optimal modification of media for the explant initiation, multiplication and rooting
4) To calculate the cost of propagation by the method of micro cloning
The object of the study is the process of the Pseudotsuga menziesii propagation by the method of micro cloning, which includes the following stages: the decontamination, initiation, multiplication, and rooting of explants.
The scientific novelty of the obtained results
On the basis of the conducted research we have improved:
1) The stepped decontamination scheme that has provided for obtaining 76% of living explants of the studied species
2) The stage of initiation of the explants harvested from the introduced plant seedlings, so that their number has increased to 89%
3) The technology of shoot multiplication, by which the highest proportion of the introduced plant explants, forming the advective micro-shoots, has been received from seedlings 91%
4) The method for rooting of the explants of the studied species providing for 83% success rate
5) The process of adaptation of the obtained clones to soil conditions, resulting in 79% of living plants
Materials and Methods
We have conducted the experiments on propagation of the Pseudotsuga menziesii genotypes by the in vitro method in two ways, using the apexes of apical and lateral buds obtained from 3-year-old seedlings (grown in the open ground in the forest nursery of Stradchivsky vocational education and training center of the National Forestry and Wood-Technology University of Ukraine) and 45-year-old plus trees growing on the territory of Hermakivskyi forestry (planning compartment-8, stratum-7) of the State-owned Enterprise ‘Chortkivskyi Forestry’ of the Ternopol Regional Forestry and Hunting Management Inspectorateas explants since February (the third decade)as in this period of the year the aboveground organs of trees are the most sterile because of frost.
The decontamination has been performed using the most common chemical reagents: C2H5OH, water with detergent, NaClO, H2O2, AgNO3. In different variants of the experiment on decontamination, we excluded one of the reagents on a rota basis. On being treated with each reagent, the explants were washed three times with sterile distilled water for 5-8 minutes. In total, there were ten variants of the experiment. 100 clones in each variant were used for this purpose:
1) Water with detergent (4 hours)+40% NaClO (5 min)+70% C2H5OH (10 sec)+2% H2O2 (5 sec)+0.2% AgNO3 (5 min)
2) Water with detergent (16 hours)+60% NaClO (5 min)+96% C2H5OH (10 sec)+3% H2O2 (5 sec)+0.3% AgNO3 (5 min)
3) Water with detergent (2 hours)+30% NaClO (5 min)+50% C2H5OH (10 sec)+1% H2O2 (5 sec)+0.1% AgNO3 (5 min)
4) 96% C2H5OH (10 sec)+water with detergent (8 hours)+50% NaClO (5 min)+70% C2H5OH (10 sec)+2% H2O2 (5 sec)
5) 96% C2H5OH (5 sec)+water with detergent (14 hours)+70% NaClO (10 min)+3% H2O2 (10 sec)+0.3% AgNO3 (10 min)
6) 96% C2H5OH (10 sec)+water with detergent (10 hours)+70% C2H5OH (10 sec)+1% H2O2 (15 sec)+0.1% AgNO3 (10 min)
7) 96% C2H5OH (10 sec)+30% NaClO (20 min)+70% C2H5OH (20 sec)+3% H2O2 (20 sec)+0.3% AgNO3 (5 min)
8) 96% C2H5OH (5 sec)+50% NaClO (10 hours)+70% C2H5OH (10 sec)+3% H2O2 (15 sec)+0.1% AgNO3 (5 min)
9) 96% C2H5OH (10 sec)+water with detergent (6 hours)+30% NaClO (15 min)+70% C2H5OH (5 sec)+0.1% AgNO3 (15 min)
10) 96% C2H5OH (15 sec)+water with detergent (12 hours)+30% NaClO (25 min)+70% C2H5OH (15 sec)+0.3% AgNO3 (15 min)
After the decontamination, the aseptic explants were passaged on the nutrient media, the test tubes were tightly closed with aluminum foil corks and placed on foam palettes. Every medium was signed and cultivated in the culture room with 16-hour photoperiod of 3 KL intensity, the temperature of 22°C/19°C (day/night) and the relative humidity of 60% (Melnychuk et al. 2000).
The passage of explants of the studied species was performed on the modified nutrient media with different ratios and concentrations of auxins (NAA, IAA, IBA) and cytokinins (BAP).
When calculating the cost of one explant of the species by the in vitro method the following expenses were taken into account: payment for utility services related to the process of propagation; payment for labour in the performance of operations on the planting material cultivation; cost of nutrient medium and reagents for the sterilization of initial explants.
Results and Discussion
The primary objective is to determine the best method of sterilization of the introduced plant explants using various chemical agents. The results of the explant decontamination on the 18th day of cultivation are shown in Fig. 1 and 2.
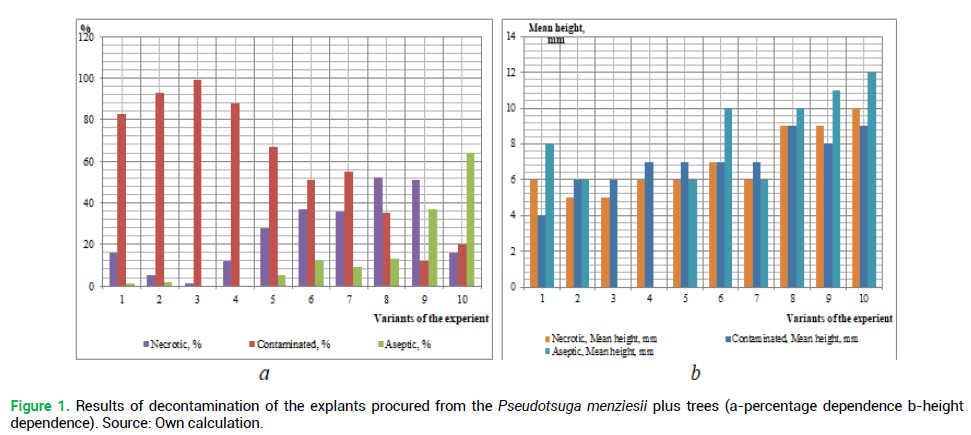
Figure 1. Results of decontamination of the explants procured from the Pseudotsuga menziesii plus trees (a-percentage dependence b-height dependence). Source: Own calculation.
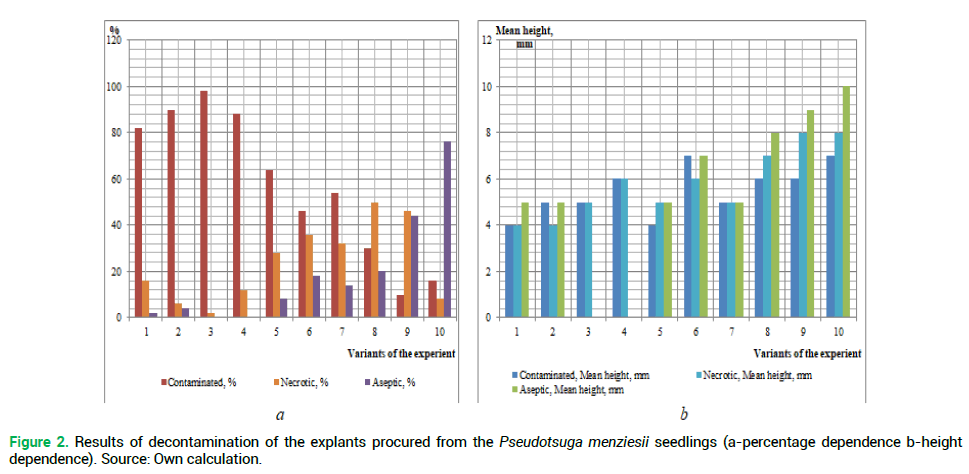
Figure 2. Results of decontamination of the explants procured from the Pseudotsuga menziesii seedlings (a-percentage dependence b-height dependence). Source: Own calculation.
Occupation of the working women: As shown in Table 2, occupation of working women was passed on Government and private job. It was found that 73% of respondent had private job and only 23% of respondent had government job. In Hostel 2, there were no respondents who were into government job.
Analyzing the obtained results of the decontamination of the studied species explants (Fig. 1 and 2), we can conclude that the obtaining of sterile original explants depends on the properly selected method of sterilization. Thus, the use of reagents of higher concentration has resulted in black discoloring (death) of the studied species explants. But low concentrations, or absence of one of the reagents, have resulted in the explant infection after 8-12 days passaging.
The highest percentage of the infected explants procured from both plus trees and seedlings of the introduced plant, was received during the sterilization in the following variants of the experiment, respectively: No 1 (83% and 82%), No 2-(93% and 90%), No 3 (99% and 98%) and No 4 (88 and 88%). This may be caused by the lack of priority treatment of explants with a solution of ethanol, which is used for removing resin droplets from planting material. A similar situation is observed in the absence of ethanol during sterilization of the above explants (the experiment variant No 5%-67% of infection rate among the explants procured from plus trees, and 64% from seedlings). A large percentage of infection was observed during the sterilization without rinsing the explants with water and detergent-No 7 (55% and 54%, respectively) and No 8 (41% and 30%) (Fig. 3). Invariant No 8 there was a large number of explants ‘burned’ by high concentrations of reagents (55%-from plus trees and 50% from seedlings).
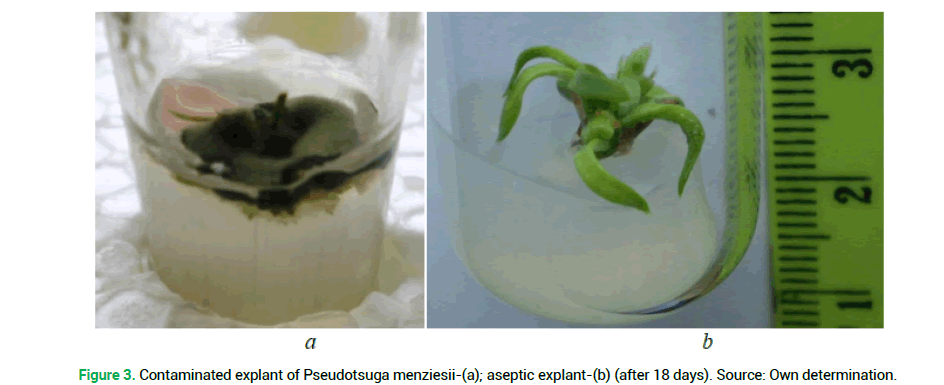
Figure 3. Contaminated explant of Pseudotsuga menziesii-(a); aseptic explant-(b) (after 18 days). Source: Own determination.
The highest percentage of the living explants procured from seedlings was obtained in variant No 10 (76%). In our opinion, this is due to the fact that juvenile plants are less contaminated. Thus, the explants from the selected plus trees (the experiment variant No 10) were of the greatest height (12 mm).
Initiation
When conducting the experiments with the culture of Pseudotsuga menziesii in the in vitro conditions, we used the modified media with different ratio and concentration of auxins (NAA, IAA, IBA) and cytokinins (BAP) in order to study their impact on the formation of callus and the initiation of the Pseudotsuga menziesii organogenesis (Tab. 1).
| The variant of the experiment | Medium | Auxins, mg/l-1 | Cytokinins, mg/l-1 | Number of initiated explants,% | |||||
|---|---|---|---|---|---|---|---|---|---|
| NAA | IAA | IBA | BAP | After 20 days | After 40 days | ||||
| from seedlings | from plus trees | from seedlings | from plus trees |
||||||
| 1 | MS | 0.5 | 0.5 | - | 0.5 | 76 | 44 | 64 | 37 |
| 2 | MS | 0.5 | - | 0.5 | 0.5 | 89 | 63 | 81 | 54 |
| 3 | МS | 8.0 | 1.0 | - | - | 5 | 3 | 1 | 1 |
| 4 | МS | - | - | - | 0.5 | 20 | 11 | 12 | 9 |
| 5 | WPM | 0.5 | 0.5 | - | 0.5 | 49 | 33 | 35 | 21 |
| 6 | WPM | 0.5 | - | 0.5 | 0.5 | 41 | 19 | 24 | 11 |
| 7 | WPM | 8.0 | 1.0 | - | - | 2 | - | - | - |
| 8 | WPM | - | - | - | 0.5 | 13 | 4 | 7 | 3 |
| 9 | LM | 0.5 | 0.5 | - | 0.5 | 67 | 41 | 55 | 31 |
| 10 | LM | 0.5 | - | 0.5 | 0.5 | 47 | 24 | 38 | 19 |
| 11 | LM | 8.0 | 1.0 | - | - | 2 | 1 | - | - |
| 12 | LM | - | - | - | 0.5 | 15 | 6 | 12 | 4 |
Table 1. Modification of nutrient media for the Pseudotsuga menziesii initiation in the in vitro conditions. Source: Own calculation.
The data of Tab. 1 shows that the best indicators of the initiation of explants procured from the introduced plant seedlings are observed in the experiment variants No 1 and No 2 on MS modified medium supplemented with auxins and cytokinins (respectively, 0.5 mg/l-1 of NAA+0.5 mg/l-1 of IAA+0.5 mg/l-1 of BAP, and 0.5 mg/l-1 of NAA+0.5 mg/l-1 of IBA+0.5 mg/l-1 of BAP). The number of initiated explants after 20 days is as follows: 76% and 89%, after 40 days-64%-81%, respectively. The significantly lower results were obtained when using the explants procured from plus trees (the experiment variants No 1 and No 2), respectively, after 20 days-44% and 63%, and after 40 days-37% and 54%.
More than 50% of the initiated explants procured from the Pseudotsuga menziesii seedlings have been obtained by using LM nutrient medium supplemented with auxins and cytokinins (0.5 mg/l-1 of NAA+0.5 mg/l-1 of IAA+0.5 mg/l-1 of BAP). The percentage of the initiated explants procured from seedlings is 67% after 20 days, and 55% after 40 days (the experiment variants No 9).
After 10-15 days from the passaging, we noticed the explant growth inhibition, and, subsequently, the complete cessation of their development on the nutrient media with the use of auxins at a concentration of 8 mg/l-1. In the latter case, the growth inhibition occurred later-after 20-22 days (the experience variants No 3, 7 and 11). The explant growth on the media without auxins is generally absent (the experience variants No 4, 8 and 12).
The experiment results show that we have succeeded in obtaining the initial callus from all types of the explants cultured on the modified nutrient media.
The results of the next stage of the in vitro propagation: the proliferation of callus with the formation of adventive buds (Fig. 4).

Figure 4. The proliferation of callus with the formation of the Pseudotsuga menziesii adventive buds. Source: Own determination.
The explants have accumulated callus phytomass for 24-30 days. After that, we made the division of callus (multiplication) for its recultivation on the modified nutrient media.
Shoot multiplication stage
After the formation of shoots on the initiated explants (4-6 weeks after initiation), they were divided into shoot apexes and node segments,and continued to be used for passaging every 4 weeks on MS and LM modified media supplemented with different ratio and concentration of auxins (NAA, IAA, IBA) and cytokinins (BAP) (Tab. 2). WPM nutrient medium is not used since its usage during the initiation in the previous experiments has resulted in the worst outcomes.
| The variant of the experiment | Nutrient medium | Supplement | Explant multiplication indicators | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAA, mg/l-1 |
IBA, mg/l-1 |
BAP, mg/l-1 |
R,% | SH, pcs. | SЕ, pcs. | |||||
| from seedlings | from plus trees | from seedlings | from plus trees | from seedlings | from plus trees | |||||
| 1 | MS | 0.5 | 0,5 | 0,3 | 11 | 9 | 2,3 | 1,1 | - | - |
| 2 | 0.5 | 0,5 | 0,5 | 20 | 7 | 2,7 | 1,5 | 0,8 | 0,4 | |
| 3 | 0,5 | 0,5 | 1,5 | 16 | 8 | 2,1 | 1,4 | - | - | |
| 4 | 0,5 | 0,5 | 1,0 | 31 | 11 | 3,3 | 2,1 | 1,5 | 1,3 | |
| 5 | 0,5 | - | 1,0 | 16 | 10 | 1,5 | 1,4 | - | - | |
| 6 | - | 0,5 | 1,0 | 18 | 4 | 2,2 | 1,0 | 0,9 | 0,5 | |
| 7 | LM | 0,5 | 0,5 | 0,3 | 56 | 21 | 3,7 | 2,7 | 1,3 | - |
| 8 | 0,5 | 0,5 | 0,5 | 43 | 22 | 6,1 | 2,9 | 1,6 | - | |
| 9 | 0.5 | 0.5 | 1.5 | 63 | 31 | 7.6 | 3.1 | 3.0 | 1.0 | |
| 10 | 0.5 | 0.5 | 0.1 | 63 | 31 | 7.6 | 3.1 | 3.0 | 1.0 | |
| 11 | 0.5 | - | 1.0 | 91 | 31 | 7.6 | 3.1 | 3.0 | 1.0 | |
| 12 | - | 0,5 | 1,0 | 18 | 8 | 2,2 | 2,1 | 0,9 | - | |
Note: R is the proportion of the explants that have formed advective micro-shoots; SH-number of new micro-shoots per explant; SE-number of new segments per micro-shoot.
Source: Own calculation.
Table 2. Indicators of the Pseudotsuga menziesii explant multiplication.
The analysis of the shoot multiplication results (Tab. 2) shows that the highest proportion of the explants forming advective micro-shoot sand the largest number of new micro-shoot per explant and new segments per microshoot have been received from the seedlings of the studied species in the experient variant No 9-63%; 7.6 pcs.; 3.0 pcs. respectively (LM modified medium supplemented with auxins and cytokinins (0.5 mg/l-1 of NAA+0.5 mg/l-1 of IBA+1.5 mg/l-1 of BAP) is used), and the experient variant No 10-91%; 7.4 pcs.; 3.6 pcs., respectively (LM modified medium supplemented with auxins and cytokinins at a concentration of 0.5 mg/l-1 of NAA+0.5 mg/l-1 of IBA+1.0 mg/l-1 of BAP is used).
The rooting of explants
Explants have been performed after the multiplication stage for 20-30 days on MS and LM nutrient media with a reduced amount of mineral salts (twice) and supplemented with only auxins (Tab. 3).
| The variant of the experience | Nutrient medium | Supplement | Number of the rooted explants,% | ||||
|---|---|---|---|---|---|---|---|
| NAA, mgl-1 |
IBA, mgl-1 |
after 20 days | after 30 days | ||||
| from seedlings | from plus trees | from seedlings | from plus trees | ||||
| 1 | 0.5 MS | 0.5 | - | 16 | 7 | 20 | 8 |
| 2 | 0.5 MS | 0.3 | 0.3 | 22 | 10 | 25 | 10 |
| 3 | 0.5 MS | - | 0.5 | 21 | 9 | 24 | 13 |
| 4 | 0.5 MS | 0.3 | - | 14 | 6 | 23 | 9 |
| 5 | 0.5 MS | 0.1 | 0.1 | 19 | 5 | 25 | 11 |
| 6 | 0.5 MS | - | 0.3 | 20 | 8 | 29 | 13 |
| 7 | 0.5 LM | - | 0.3 | 50 | 36 | 75 | 53 |
| 8 | 0.5 LM | 0.3 | 0.3 | 47 | 35 | 58 | 45 |
| 9 | 0.5 LM | 0.3 | - | 18 | 9 | 24 | 14 |
| 10 | 0.5 LM | - | 0.5 | 71 | 51 | 83 | 59 |
| 11 | 0.5 LM | 0.1 | 0.1 | 31 | 18 | 37 | 27 |
| 12 | 0.5 LM | 0.3 | - | 23 | 10 | 26 | 17 |
Table 3. Indicators of rhizogenesis of explants of the studied species. Source: Own calculation.
According to the obtained data (Tab. 3), the highest percentage of rooting of the studied species explants was observed in the experiment variant No 7 (75%) on 0.5 LM medium supplemented with 0.3 mg l-1 of IBA, and in the experiment variant No 10 (83%) on 0.5 LM medium supplemented with 0.5 mg/l-1 of IBA. The lowest results of rooting were obtained in the explants cultured on0.5 MS nutrient medium, or without the supplement of IBA to the medium. Thus, it should be noted that the percentage of rooting of the explants procured from plus trees (after 30 days the percentage of rooting amounted to 8%-59%) in all variants of the experience was lower compared to the explants procured from seedlings (after 30 days the percentage of rooting amounted to 20%-83%).
The final stage of the in vitro propagation of Pseudotsuga menziesii is the successful adaptation of the obtained clones to soil conditions. The explants with a well-developed root system were removed from tubes with forceps, cleared out of agar remnants under distilled water, and planted out in cassettes with the sterilized substrate. The two types of the substrate with neutral or slightly acid reaction were used: soddy soil with sand (1:1) and soddy soil with sand and peat (1:1:1). The adaptation of clones of the studied species was conducted in the conditions of the culture room. In order to protect plants from fungal diseases, the substrate was pre-treated with a solution of fundazol (5%) and potassium permanganate (1.2%)-2-3 days prior to planting.
The cultivation of the clones procured from the studied species seedlings for 60 days resulted in obtaining 51% and 79% of living plants, respectively (Fig. 5). The cultivation of the clones procured from plus trees of the introduced plant for 60 days resulted in obtaining 43% and 52% of living plants, respectively. The established seedlings were transferred from cassettes with the substrate to the open ground on the specially prepared areas.

Figure 5. Adaptation of regenerated Pseudotsuga menziesii to the substrate: (а)-a mixture of peat, sand, and loam in the ratio of 1:1:1; (b)-sod-weak podzolic sandy loam soil. Source: Own determination.
The study of the stages of the introduced plant experimental morphogenesis by the vitro method on all levels of the organization, given the complexity of the propagation of coniferous plants by the method of micro-cloning, is aimed at creating an effective technology of propagation of plants that can be used for commercial purposes. This method of propagation of the species resistant to unfavorable climatic conditions and the response to biotic factors can be used for mass cultivation, but at the same time, it is worth considering the cost during the above-mentioned study on Pseudotsuga menziesii.
In general, the calculation flow-chart of the introduced species propagation by the in vitro method includes the following technological operations: procurement of explants (buds); sterilization of initial explants; preparation of medium with pouring; ware provision; explant planting; transplantation of explants for rooting; preparation of substrate with dispersal; clone potting; watering (1 time a day with 10 liters of water per month); determination of plants for growing completion; wrapping for winter; unwrapping, 8-time seedling weeding (loosening with weed removal). The cost of propagation of one thousand explants of the studied species by the in vitro method was 756 US dollars.
For example, the analysis of calculations carried out in the experiments on the Pseudotsuga menziesii propagation by seeds in the Western Forest-steppe of Ukraine shows that the cost of cultivation of one thousand seedlings of the introduced plant has amounted to 7.55 US dollars. In this case, the calculation flowchart for growing 1,000 Pseudotsuga menziesii seedlings included the following technological operations: soil tillage for sowing seed (peat-manure compost spreading, autumn under winter plowing, spring soil disking in 2 tracks, early spring plowing, cultivation with simultaneous harrowing (8 times), organic fertilizer application, autumn plowing, soil polishing before planting of seedlings, row layout under the cord with the soil loosening and leveling); seed preparation for sowing (pre-sowing seed treatment (0.5% solution of ‘potassium permanganate’), seed drying in shade before sowing); seed sowing (seed sowing in rows with the marking and deepening of furrows to a depth of 1.0-1.5 cm with the substrate embedding and compaction (row length-100 m, number of rows-2), crop watering (1 time every four days, 10 l per 10 m of row)-23 times during the growing period, 8-time seedling weeding (loosening by hand with weed removal). The second year of seedling growing included: crop watering (1 time every four days, 10 l per 10 m of a row)-23 times during the growing period (4600 l of water), 8-time seedling weeding (loosening with weed removal).
Accordingly, the cost of propagation of one thousand seedlings of the studied species by the generative method is 29 times less compared to the in vitro propagation.
Conclusion
The success of Pseudotsuga menziesii propagation in the in vitro conditions depends on the properly chosen methods of micro-cloning propagation of the species, namely:
1) At all stages of the introduced propagation in the in vitro conditions, the explants procured from 3-yearold seedlings are the most capable of morphogenesis
2) It is expedient to carry out the decontamination of initial explants in the following sequence: 96% with aqueous solution of 25 for 15 sec; with flow 20 with detergent for 12 hours; 30% with aqueous solution of NaCIO for 25 min; 70% with aqueous solution of 25-15 sec; 0.3% with aqueous solution of AgNO3 for 15 min; subject to triple rinsing of the selected meristems with distilled water after each reagent. 76% of aseptic explants procured from seedlings were obtained
3) MS nutrient medium supplemented with auxins and cytokinins of 0.5 mg/l-1 of NAA+0.5 mg/l-1 of IBA+0.5 mg/l-1 of BAP should be used for initiation of the studied explants. This will provide for the greatest number of initiated explants (81% after 40 days of cultivation)
4) LM solid nutrient medium supplemented with auxins and cytokinins at a concentration of 0.5 mg/l-1 of NAA+0.5 mg/l-1 of IBA+1.0 mg/l-1 of BAP should be used for multiplication of initiated explants. The use of this medium ensures the obtaining of 91% of explants with advective micro-shoots; 7.4 new micro-shoots per explant and 3.6 new segments per micro-shoot
5) The most suitable nutrient for rooting of the introduced plant micro-shoots is 0.5 LM supplemented with auxin of 0.5 mg/l-1 of IBA. Under these conditions, the percentage of rooting on the 30th day amounted to 83%
6) It is expedient to carry out the adaptation of the studied regenerated species to soil conditions under the culture room in soddy soil with sand and peat (1:1:1), which provides for obtaining 79% of adapted plants after 60-day cultivation. In this case, 2-3 days before planting, the substrate should be pre-treated with a solution of fundazol (5%) and potassium permanganate (1.2%) to protect plants from fungal diseases
The in vitro propagation of the studied species for industrial purposes should be carried out if all the stages of work are automated. High prices for substances required for the sterilization of explants and the preparation of modified nutrient media are connected with the fact that most of them are imported.
References
- Dunstan D.I., Lashta D.P., Kikcio S.I., Thorpe T.A. 1992. Factors affecting recurrent shoot multiplication in vitro cultures of 17-to 20-year-old Douglas fir trees. In Vitro Cell and Develop Bio-Plant. 28: 33-38. https://link.springer.com/article/10.1007/BF02632190
- Ramage C.M., Williams R.R. 2002. Mineral nutrition and plant morphogenesis. In Vitro Cellular and Develop Bio-Plant. 38: 116-124. https://link.springer.com/article/10.1079/IVP2001269
- Pullman G.S., Johnson S., Bucalo K. 2009. Douglas fir embryogenic tissue initiation. Plant Cell, Tissue and Organ Culture. 96: 75-84. https://www.academia.edu/9541671/Douglas_fir_embryogenic_tissue_initiation
- Gupta P.K., Durzan D.J. 1985. Shoot multiplication from mature Douglas-fir and sugar pine. Plant Cell Rep. 4: 177-179. https://link.springer.com/article/10.1007/BF00269282
- Winton L.L., Verhagen S.A. 1977. Shoots from Douglas-fir cultures. Canad J Bot. 55: 1246-1250. https://www.nrcresearchpress.com/doi/abs/10.1139/b77-144?journalCode=cjb1#.XcLZUZozY2w
- Al-Talib K.H., Torrey J.G. 1959. The aseptic culture of isolated buds of Pseudotsuga taxifolia. Depart Bot, Uni California, Berkeley. 34: 630-637. http://www.plantphysiol.org/content/34/6/630
- Traore A., Xing Z., Bonser A., Carlson J. 2005. Optimizing a protocol for sterilization and in vitro establishment of vegetative buds from mature Douglas fir trees. School Forest Resour, Pennsylvania State Uni, Horticult Sci. 40: 1464-1468. http://agris.fao.org/agris-search/search.do?recordID=US201301019328
- Douglas D. 1914. Journal kept by David Douglas during his travels in North America 1823-1827. William Wesley and Son, London. 373. https://archive.org/details/journalkeptbydav00dougiala/page/n7
- Durzan D.J., Gupta P.K. 1987. Somatic embryogenesis and polyembryogenesis in douglas-fir cell suspension cultures. Plant Sci. 52: 229-235. https://www.sciencedirect.com/science/article/pii/0168945287900562
- Gupta P.K, Durzan D.J. 1987. In vitro establishment and multiplication of juvenile and mature Douglas-fir and sugar pine. Acta Horticult. 212: 483-488. https://doi.org/10.17660/ActaHortic.1987.212.74
- Konnert M., Ruetz W. 2006. Genetic aspects of artificial regeneration of Douglas-fir (Pseudotsuga menziesii) in Bavaria. Europ J Forest Res. 125: 262-270. https://www.semanticscholar.org/paper/Genetic-aspects-of-artificial-regeneration-of-in-Konnert-Ruetz/4185c84ef888e5d3af5853b81cf6c860ed0c8dfe
- Guz M.M., Yaroshcuk R.A., Нreсhanуk R.M. 2011. Genetic resources Douglas Menzisa (Pseudotsuga menziesii (Mirb.) Franco) in Ukraine. Scient Bull Ukrainian Nat Forest Uni. 21: 15-22. https://cyberleninka.ru/article/n/genetichni-resursi-psevdotsugi-menzisa-pseudotsuga-menziesii-mirb-franco-v-ukrayini
- Guz M.M., Yaroshcuk R.A. 2012. Seed potential of menzies pseudotubes and sowing qualities of seeds of the species in the western forest-steppe of Ukraine. Scient Bull Ukrainian Nat Forest Uni. 22: 9-14. https://cyberleninka.ru/article/n/nasinniy-potentsial-psevdotsugi-menzisa-ta-posivni-yakosti-nasinnya-vidu-v-umovah-zahidnogo-lisostepu-ukrayini
- Yaroshcuk R.A. 2013. Featuresof Pseudotsuga Menziesii (Mirb.) francoin artificial forest plantations west forest-steppe Ukraine.Scient Bull Ukrainian Nat Forest Uni. 23: 79-84. https://cyberleninka.ru/article/n/osoblivosti-poshirennya-psevdotsugi-menzisa-pseudotsuga-menziesii-mirb-franco-u-shtuchnih-lisovih-nasadzhennyah-zahidnogo-lisostepu
- Morris, J.W., Doumas P., Morris R.O., Zaerr J.B. 1990. Cytokinins in vegetative and reproductive buds of Pseudotsuga menziesii. Plant Physiol. 93: 67-71. https://www.ncbi.nlm.nih.gov/pubmed/16667467
- Ritchie G.A., Tanaka Y., Duke S.D. 1992. Physiology and morphology of Douglas-fir rooted cuttings compared to seedlings and transplants. Tree Physiol. 10: 179-194. https://www.ncbi.nlm.nih.gov/pubmed/14969868
- Ritchie G.A., Keeley J.W., Ward P.A. 1997. Effects of shade and root confinement on the expression of plagiotropic growth in juvenile-origin Douglas-fir rooted cuttings. Canad J Forest Res. 27: 1142-1145. https://www.ncbi.nlm.nih.gov/pubmed/11540948
- Melnychuk M.D., Novak T.V., Kunakh V.O. 2003. Plant biotechnology. Kyiv: Polihraf Konsaltynh. 250. https://www.twirpx.com/file/580356/