Research - Modern Phytomorphology ( 2021) Volume 15, Issue 4
Anatomy, morphology, palynology and nutlet micromorphology of the rediscovered Turkish steno-endemic Stachys longiflora Boiss. and Bal. (Lamiaceae)
Suleyman Dogu*Suleyman Dogu, Department of Animal and Plant Production, Necmettin Erbakan University, Meram Vocational School, Konya, Turkey, Email: suleymandogu@gmail.com
Received: 28-Mar-2021 Accepted: 06-Jul-2021 Published: 14-Jul-2021, DOI: 10.5281/zenodo.5801179
Abstract
Stachys longiflora Boiss. & Bal, previously known only from the type gathering is a steno-endemic species from Turkey. The species was firstly collected by Balansa from Mersin-Guzeldere in 1855 and described by Boissier and Balansa in 1859. It was not collected again until 2011, when the author found it in Guzeldere (Mersin). Therefore, S. longiflora is treated under “Data Deficient” DD category in the Turkish Red Data Book. In this study, some anatomical and micromorphological characters, observations and measurements of S. longiflora are presented for the first time. The diagnostic morphological, anatomical, nutlet micromorphological and palynological characters of this steno-endemic species are discussed. Morphological characteristics of corollas, calyces, leaves, and stems are useful for specific delimitation in Stachys. Anatomical characters such as the shape of sclerenchymatic tissue and presence or absence of sclerenchymatic tissue in stems, mesophyll structures in leaves are of taxonomic significance. Moreover, the presence or absence of nutlets trichomes, shape and sculpturing pattern of nutlets are diagnostic characters. The emended and expanded description, ecology and phenology of this steno-endemic species are also presented along with its revised conservation status.
Introduction
Stachys L. (Lamiaceae, subfamily: Lamioideae) a subcosmopolitan genus that is mainly centered in the temperate regions of Mediterranean and Southwest Asia and, secondarily, in North and South America and South Africa. It is one of the largest genera of Labiatae which contains about 370 species (435 taxa) around the world (Harley et al. 2004; Govaerts 2019). There are two centers of the genus in terms of the number of species. One of them is Southern and Eastern Anatolia, Caucasia, Northwestern Iran, Northern Iraq and the other is Balkan peninsula (Bhattacharjee 1974, 1980). Many species of this genus are distributed in rocky areas, especially on limestone rocks (Bhattacharjee 1974, 1980; Harley et al. 2004).
In the Flora of Turkey, Stachys was revised by Bhattacharjee (1982). Since then, 20 new species were described from Turkey. Stachys has 91 species (118 taxa) belonging to 15 sections and 2 subgenera in Turkey. Of the 118 taxa, 54(45.7 %) are endemic to Turkey (Bhattacharjee 1982; Davis et al. 1988; Duman 2000; Dinc & Dogan 2006; Ilcim et al. 2008; Akcicek 2010; Yildirimli 2010; Yilmaz et al. 2010; Dirmenci et al. 2011; Ozhatay et al. 2011; Dinc & Dogu 2016; Akcicek et al. 2016). The endemic taxa are mostly eastern Mediterranean elements. Turkey as a center of diversity of Stachys contains about 91 species (Celep & Dirmenci 2017).
Representatives of Stachys sect. Fragilicaulis R. Bhattacharjee distributed only Turkey, Iran, Iraq and Southern Caucasus. In Turkey, Stachys sect. Fragilicaulis which is divided into two subsection (Fragiles Rech. f., Multibracteolatae R. Bhattacharjee) is composed of 22 taxa, 15 of which are endemic. Members of Stachys subsect. Fragiles of which seven are endemic taxa were indicated as chasmophilous (dwelling in rock crevices or joints) and they are all distributed in the Taurus mountain range in South Turkey. (Bhattacharjee 1982; Rechinger 1982; Davis et al. 1988; Duman 2000).
Stachys longiflora Boiss. & Bal. (subsect. Fragiles), a local endemic species for Turkey, was firstly collected by Balansa from the northern part of Mersin province ‘in speluncis faucis Guzel Dere supra Sedichig in Cilicia littorali prope Mersina’ in 1855 and described as a new species by Boissier and Balansa in 1859, but since that time any specimen has not been collected. Because the local name ‘Guzel Dere’ has been changed. The local name ‘Guzel Dere’ was only known by the vicinity people in that area, especially by the elderly. Nowadays, that area is known as ‘Kizil Dere’. In 2011, in the scope of the taxonomical studies on the species belonging to subsect. Fragiles, some interesting specimens were collected on the field trip to Mersin-Kizil Dere region by the author.
The specimens collected from Kizil Dere (Mersin) were checked with descriptions given in both the “Flora of Turkey” and in the original description, also specimens compared with the herbarium specimens which kept in ANK, AEF, B, BM, E, G, GAZI, H, HUB, ISTE, ISTF, K and KNYA herbaria. As a result, the author identified the specimens collected from Kizil Dere-Mersin in 2011, as S. longiflora. Thus, S. longiflora, which had not been collected for the past 156 years, was rediscovered. The author has also determined that the local name ‘Guzel Dere’ is currently being used as ‘Kizil Dere’.
This study aims to document the rediscovery of a population of the species, to give a detailed account of the anatomical, morphological, pollen and nutlet micromorphological characteristics of S. longiflora, and to evaluate their significance for the taxonomy of the genus.
Materials and Methods
The specimens that are belonging to S. longiflora were collected from Kizil Dere district of Mersin province. The collecting locality of the specimens is Turkey, C5 Mersin: Işıktepe village, Kizil Dere, limestone crevices and cave mouth, 250 m-350 m, 36º52´36 35N, 34º33´14 74E, 17 July 2011, S. Dogu 2730 and 07 October 2011, S. Dogu 2740, ibid.
Fifteen individuals of the S. longiflora were collected in Kizil Dere district, during the flowering and fruiting stage (Fig. 1). The voucher specimens were prepared according to standard herbarium techniques and stored in the Herbarium of the Selçuk University (KNYA). Other samples collected from natural habitats were fixed in 70% ethyl alcohol for anatomical studies. The studies were performed using 15 samples for species. Anatomical examinations, cross-sections of the stem and leaves were made together with the adaxial and abaxial surfaces of the leaves. An average of 25 slides was prepared for each type of section and stained with basic fuchsin for easy identification of the tissues. Well-stained sections covered with glycerin gelatin were made into permanent slides according to Vardar (1987). The stained preparations were examined with an Olympus BX50 microscope and photographed by Cameram imaging apparatus.
Some of the herbarium samples were used in the studies on pollen and nutlet morphological studies. The pollen morphology studies were made under the light and Scanning Electron Microscope (SEM). The studies of a Light Microscope (LM) were made on preparates which were prepared according to the Wodehouse (1935) technique. For this, the material on the microscope slide was mixed by a needle to distribute the pollens with dripping fused glycerin-gelatine with basic fuchsin. The preparate was preserved as covering with coverslip over it in which microscope slide was placed at the bottom until the gelatine freezes. Each of the measurements was made by pollen morphology with the help of ocular and objective micrometer on 30 pollen. For the SEM studies, pollen grains were put on aluminum stabs and were covered with gold using ZEISS LS-10 gold coating apparatus on low-vacuum mode at about 20 nm in thickness to observe their surface structures. Pollen grains were examined and micrographs were obtained at magnifications ranging from ×3000-×15,000 with a JEOL-JEM 2100 scanning electron microscope at ILTEK (Advanced Technology Search and Application Centre of Selçuk University, Konya) to determine the exine ornamentation. The terminology of pollen morphology is arranged according to Punt et al. (1994).
The studies about the nutlet morphology were made by stereomicroscope and SEM. Also, the mature nutlets were covered with gold on aluminium stab for determining the surface ornamentation for SEM studies. The measurements about the nutlet were made by the stereo binocular microscope on 20 mature nutlets.
Photographs of the preparates were taken with an Olympus BX-50 microscope. The stomatal index and stomatal index rate were calculated as described by Meidner and Mansfield (1968).
Results
Stachys longiflora Boiss. & Bal.
Type: TURKEY. C5 Mersin: in speluncis faucis Güzel Dere supra Sedichig in Cilicia littorali prope Mersina, 02 × 1855, Balansa 598. (holo: G!; iso: E!, K!).
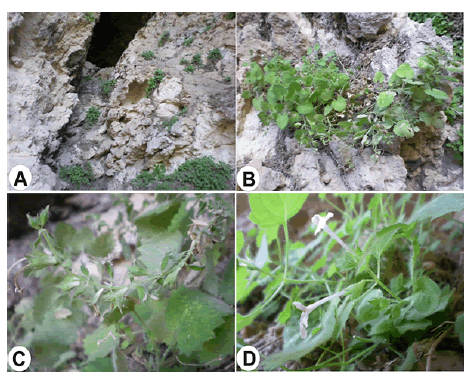
Description: Perennial suffruticose herbs. Flowering stems 15 cm–65 cm, pendent, fragile at the base, usually branched from base, very densely patent-pilose with short glandular and long eglandular hairs. Cauline leaves orbicular to widely ovate, (1.2–6.3) cm × (1–5.7) cm, margin dentate to crenate-dentate, apex narrowly to widely acute, usually cordate, rarely subcordate at base, thin and herbaceous, pilose, petiolate; petiole 1 cm–5 cm, eglandular pilose and short glandular hairs. Floral leaves similar to cauline leaves but smaller, (0.5–3.5) cm × (0.4–3.1) cm, margin dentate, apex acute, usually turuncate to subcordate, rarely widely cuneate at base, sparsely pilose, petiolate to subsessile; petiole 1 mm–13 mm. Verticillasters usually remote throughout, rarely uppermost approximate, 0.4 cm–2.1 cm distance, 1–2 flowered, ebracteolate. Pedicels 1 mm–7 mm, sparsely pilose with, capitate and peltate glandular hairs, rigid, erecto-patent. Calyx ± regular, infundibular to campanulate, mouth without hairy ring, 0.7–15 mm at flowering, up to 18 mm and scarcely broadening at fruiting, herbaceous, densely glandular and eglandular pilose with minutely sessile glands; teeth ± equal, 1/2 × tube, triangular-ovate to triangular lanceolate, glabrescent and spinescent tipped, mucro 0.5 mm–3 mm. Corolla white to pinkish, 20 mm–33 mm; lips streaked and spotted with pink inside, glandular with rarely sparsely pilose with peltate glands outside, minutely hairy inside; upper lip 2.1 mm and lower one 4.3 mm long; tube exserted, very narrowly cylindrical, exannulate, minutely hairy upper half inside and outside, Nutlets elongate, bright brown, elliptic-oblong, apex rounded (Fig. 1).
Figure 1: Stachys longiflora Boiss. & Bal. A: Natural habitat; B: General view in natural habitat; C: Inflorescence; D: Flowers. (All photographs by S. Dogu.)
Phenology, habitat and ecology
Stachys longiflora is flowering in July-October and fruiting in August–November. This species grows on limestone crevices and mouths of caverns between attitudes of 250 m and 350 m. Other species in its habitat are Teucrium odontites Boiss. & Balansa, T. lamiifolium d’Urv., T. kotschyanum Poech, Stachys rupestris Bentham, Origanum majorana L., Scutellaria rubicunda Hornem subsp. pannosula (Rech.f.) J.R.Edm., Ficus carica L., Pistacia terebinthus L., Hypericum lanuginosum Lam., Styrax officinalis L.
Anatomical characteristics
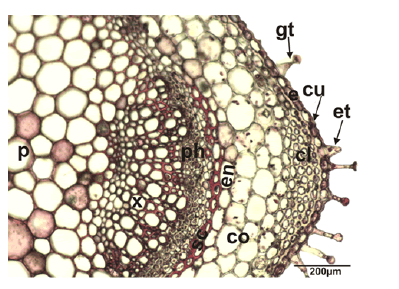
Stem anatomy: General view of the stem is rectangular in the transverse section. The epidermis is covered by a thin and smooth cuticle and its consists of uniseriate squarish, rectangular, or oval cells. The epidermis contains sparsely eglandular and glandular trichomes. The collenchyma tissue is located at the corners of the stem and composed of 3–5 layered cells whose walls are thickened. There is a cortex layer between the collenchyma and endodermis tissue which is composed of 3–5 layers of rectangular and irregularly oval parenchymatic cells. The endodermis is regular, single-layered and composed of quadrate, subglobose and ovoid cells. However, endodermis tissue cells did not show a significant differentiation from the cortex parenchyma cells. Fibres of sclerenchyma are formed as bouquets which consist of 2–3 sheets and are seen as sickle in the corner. Vascular bundles are next to each other. The size of the vascular bundles at the corners is distinctly larger than the others. In the vascular tissue, xylem and phloem elements can be distinguished. The cambium between the xylem and phloem is inconspicuous. In the center of the stem, the pith is wide and filled with hexagonal, slightly pentagonal and orbicular parenchymatous cells with intercellular spaces (Fig. 2).
Figure 2: Transverse section of the stem of Stachys longiflora, co: cortex; cl: collenchyma; cu: cuticle; e: epidermis; ph: phloem; p: pith region; sc: sclerenchyma; x: xylem; gt: glandular trichome; et: eglandular trichome.
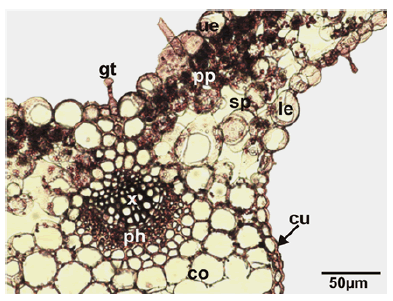
Mesophyll anatomy: In the cross-section, the lamina and mid-rib are seen to be covered by a regular layer of the epidermis. Both epidermis is composed of ovoid, quadrate and rectangular cells and both surfaces are covered with a thick layer of cuticle. The thickness of both epidermis cuticles is nearly equal. There are glandular and non-glandular trichomes on both epidermis layers surrounding the abaxial and adaxial surfaces. Both leaf surfaces are covered by glandular and non-glandular hairs of the same type as seen on the stem. Epidermal cells whose thickened external walls are seen on both sides of the leaf. The leaf is dorsiventral and hypostomatic type. The mesophyll is composed of circular spongy parenchyma and cylindrical palisade parenchyma cells. Palisade parenchyma under the upper epidermis is composed of 1–3 layered cylindrical cells arranged perpendicular to the leaf lamina. The palisade parenchyma occupies about 60%–65% of the mesophyll. The spongy parenchyma cells are 2–3 layered under the palisade parenchyma tissue. There are more intercellular spaces between the spongy parenchyma cells. The mesophyll is thicker around the central vein. Mid-rib is triangle shaped and has a 3–4 layered collenchyma located below lower epidermal cells. The collateral vascular bundle is located in the central part of the midvein. Vascular bundles in the midvein are larger than the lateral ones and all vascular bundles are surrounded by a parenchymatic bundle sheath. The xylem layer is just below the collenchyma. 1–2 layered parenchyma and 3–4 layered collenchyma are located under the phloem (Fig.3).
Figure 3: Transverse section of the leaves of Stachys longiflora. cu: cuticle; gt: glandular trichome; le: lower epidermis; ue: upper epidermis; pp: palisade parenchyma; sp: spongy parenchyma; co: collenchyma; x: xylem; ph: phloem.
Leaf surface anatomy: The leaf surface sections showed that the leaves are hypostomatic (the upper surface of the leaf lacks stomata) with diacytic type stomata and the wall of the upper epidermal cells is thicker than the one of the lower epidermal cells. The number of stomata is 98 per mm2 ± 4 per mm2, and the size of the epidermal cells is 314 per mm2 ± 7 per mm2 on the lower leaf surface. The stomatal index is 20.6. The stoma cells form a row below the epidermis (xeromorphic type). The sphaerocrystals were observed in some upper epidermal cells of leaves (Fig. 4).
Figure 4: Upper and lower surface sections of the leaves of Stachys longiflora. st: stoma; ec: epidermal cell; scr: sphaerocrystal.
Pollen Characteristics
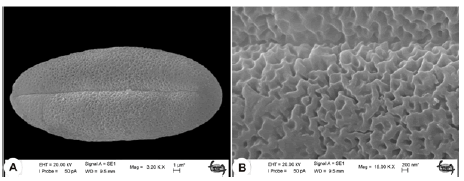
Pollen grains of the S. longiflora are single, isopolar and tricolpate. The mean of a polar axis (P) and equatorial axis (E) is 33.12 µm ± 4.33 µm (29.84 µm–36.65 µm) and 15.0 µm ± 3.12 µm (13.91 µm–18.03 µm). The shape of pollen grains is prolate to perprolate (P/E=1.87 µm–2.4 µm). The colpi length (Cl) and colpus width (Clg) are 28.75 µm ± 3.98 µm and 2.25 µm ± 0.18 µm. The outline is elliptic in the equatorial view. The sculpturing of the exine in S. longiflora is micro-reticulate with perforations. The exine thickness is between 1.20 µm and 1.35 µm. The Intine thickness is 0.3 µm–0.5 µm. The ornamentation is retipilate. The width of the muri is 0.5 µm-0.6 µm and that of lumina is 0.8 µm–1.4 µm (Fig. 5).
Figure 5: SEM micrographs of the pollen of Stachys longiflora. A: equatorial view; B: Exine sculpturing (S Dogu 2730).
Nutlet Micromorphology
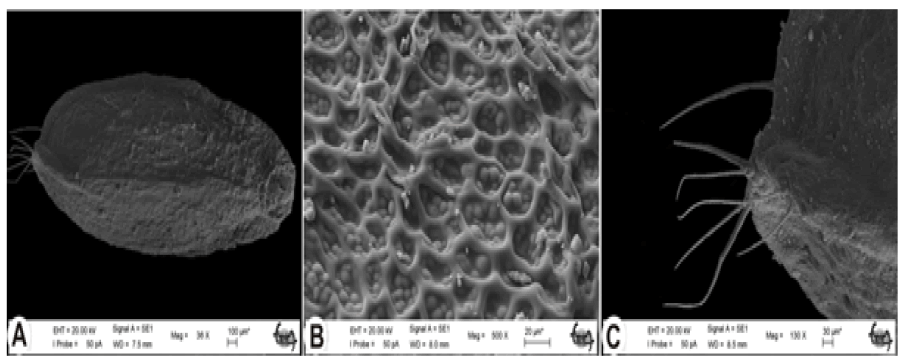
Nutlets are elliptic-oblong-shaped. The apex of trigonous nutlets is obtuse in S. longifolia. Areoles are small and circular or square. Nutlets are bright brown and size ranges between 2.89 mm and 3.57 mm in length and 1.27 mm and 1.43 mm in width. Nutlet surfaces are reticulate-papillate. The reticulate pattern consists of small polygonal cells with papillate. The apex of the nutlet is long-haired which consists of 1–4 cells (Fig. 6).
Figure 6: SEM micrographs of the nutlet of Stachys longiflora. A: general appearance; B: surface ornamentation; C: non-glandular trichomes (S Dogu 2740).
Conservation Status
Stachys longiflora has not been collected since 1855, so it was treated as ‘Data Deficiency’ (DD) by Ekim et al. (2000). During a field study conducted in 2011, the author found the species at the Kizil Dere in Mersin province. According to the author's field observations, its current conservation status has been re-evaluated. The population size covers about 100 m by 50 m and is bisected by a paved road from Mersin to Asagi Keslik village. According to the IUCN criteria guidelines for their application (IUCN 2014), taking the criterion B1ab (iii) (EOO<100 km2, fragmented locations due to road and declining area of occupancy) and D1 (number of mature individuals less than 100 in a population), the author strongly propose that S. longiflora should be recognized as Critically Endangered (CR) (Tab. 1 and Tab. 2).
| Present data ( S Dogu 2730, 2740) | Flora of Turkey (Bhattacharjee 1982) | |
|---|---|---|
| Stem (cm) | 15-65 | 44-50 |
| branched from base | - | |
| Cauline leaves (cm) | 1.2-6.3 × 1-5.7 | 3-6 × 2.5-5.5 |
| orbiculare to widely ovate | ovate to ovate-orbicular | |
| margin dentate to crenate-dentate | margin broadly dentate | |
| apex narrowly to widely acute | apex acute | |
| usually cordate, rarely subcordate at base | cordate at base | |
| sparsely pilose | - | |
| Petiole (cm) | 1-5 | 3-4.5 |
| Floral leaves (cm) | 0.5-3.5 × 0.4-3.1 | - |
| margin dentate | - | |
| apex acute | - | |
| usually turuncate to subcordate, rarely widely cuneate at base | cuneate to truncate at base | |
| sparsely pilose | - | |
| Petiole (mm) | 1-13 | 2-9 |
| Inflorescence (cm) | 5-21 | - |
| Verticillasters | usually remote throughout, rarely uppermost approximate | remote |
| 0.4 cm-2.1 cm distant | 0.7 cm-1.2 cm distant | |
| Pedicel (mm) | 1-7 | 1-3 |
| Calyx | infundibular to campanulate | infundibular |
| Calyx (in flower, mm) | 0.7-15 | 10-13 |
| Calyx (in fruit, mm) | up to 18 | - |
| Calyx | densely glandular and eglandular pilose with minutely sessile glands | - |
| Calyx teeth | triangular-ovate to triangular lanceolate | - |
| Mucro (mm) | 0.5-3 | 0.5-1 |
| Corolla (mm) | 20-33 | 22-32 |
| white to pinkish | purple | |
| Lips | spotted with pink inside, glandular with rarely sparsely pilose with peltate glands outside | - |
| Upper lip (mm) | 1.9-2.1 | - |
| Lower lip (mm) | 4.0-4.3 | - |
Table 1. Morphological characters of Stachys longiflora based on the present study and ‘Flora of Turkey’.
| S. longiflora (Author data) | S. pseudopinardii (Bhattacharjee 1982)) | |
|---|---|---|
| Stem (cm) | brached from base, 15-65 | unbrached, 30-55 |
| densely patent-pilose | sparsely patent-pilose | |
| Cauline leaves (cm) | 1.2-6.3 × 1-5.7 | 1-5 × 0.8-4.5 |
| apex narrowly to widely acute | apex obtuse | |
| Petiole (cm) | 1-4 | 2-5 |
| Floral leaves (cm) | 0.5-3.5 × 0.4-3 | 0.5-3 × 04-2.8 |
| usually truncate to subcordate, rarely widely cuneate at base | cordate to cuneate at base | |
| Petiole (cm) | 0.1-1.3 | 0.2-1.2 |
| Verticillasters | 1-2 flowered | 4-6 flowered |
| Pedicels (mm) | 1-7 | 2-2.5 |
| Calyx | ± regular | sub-bilabiate |
| Calyx teeth | ± equal | unequal |
| Corolla (mm) | 20-33 | 20-22 |
Table 2. Comparison of morphological characters of Stachys longiflora and closely related species.
Discussion
Some Stachys taxa are sometimes highly polymorphic and morphological differences between similar species are often unclear. Particularly, due to the large differences in macromorphological characters under different ecological conditions, taxonomy of Stachys is very difficult (Abu-Asab & Cantino, 1994). Based on gross morphological characters, taxonomic delimitation and identification of species in the subsect (Rechinger 1982).
Taxa of the subsect. Fragiles are suffrutescent saxatile perennial herbs without basal rosettes. Flowering stems are pendent and below fragile. Indumentum is patent-pilose. Leaves are orbicular to ovate and ± cordate at base. Verticillasters are (2–)6–10(–16) flowered. Bracteoles are a few, inconspicuous and setaceous. Calyces are almost infundibular to subcampanulate, teeth triangular to triangular–lanceolate and softly spinescent at apex. The Corolla tube is long and exserted from the calyx tube. Nutlets are oblong-elliptic, apiculate and unwinged (Bhattacharjee 1980). Stachys longiflora exhibits these morphological features in general. Therefore, its taxonomic placement is correct in the subsect Fragiles.
Evaluation of obtained results revealed that stem, leaf and corolla characteristics of S. longiflora were found to be more variable than reported in ‘Flora of Turkey’ (Bhattacharjee 1982). Observations and measurements of some morphological characters of S. longiflora are presented here for the first time (Tab. 1). Bhattacharjee (1982) stated that S. longiflora was closely allied to Stachys pseudopinardii Bhattacharjee and Hub.-Mor., but a critical examination of S. longiflora reveals striking differences (Tab. 2). The stems of S. longiflora are brached from base, taller and densely patent-pilose, whereas those of S. pseudopinardii are unbrached from base, short and sparsely patent-pilose. The cauline leaves of S. longiflora are 1.2 cm-6.3 cm × 1 cm-5.7 cm and apex narrowly to widely acute, but those of S. pseudopinardii are 1 cm–5 cm × 0.8 cm–4.5 cm and apex obtuse. The floral leaves are 0.5 cm–3.5 cm × 0.4 cm-3 cm and usually truncate to subcordate, rarely widely cuneate at the base in Stachys longiflora, 0.5 cm-3 cm × 04 cm–2.8 cm and cordate to cuneate at the base in S. pseudopinardii. The verticillasters of S. longiflora are 1–2 flowered, whereas those of S. pseudopinardii are 4–6 flowered. Pedicels of S. longiflora are 1 mm–7 mm, but those of S. pseudopinardii are 2 mm–2.5 mm. The calyx of S. longiflora is ± regular and teeth ± equal, whereas those of S. pseudopinardii are sub-bilabiate and teeth unequal. Corolla of S. longiflora are 20 mm–33 mm, but those of S. pseudopinardii is 20 mm–22 mm (Tab. 2).
Metcalfe and Chalk (1950) stated that the stems of many genera and species of the family Lamiaceae are rectangular and the collenchymatic tissue covers a broad area at the corners, and a developed scleranchymatic tissue surrounds the vascular tissue. Stem of S. longiflora showed the typical four-angled and structure of Lamiaceae members, covered with a one-layered epidermis with trichomes, well-developed collenchyma at the angles of the stem and ring shaped sclerenchymatic tissue outside the vascular tissue. The same observations were reported during the anatomical studies on other family Lamiaceae species in Turkey (Uysal 1991, 2002, 2003; Kaya et al. 2000; Dinc & Ozturk 2008; Kahraman et al. 2009, 2010a, 2010b; Erdogan et al. 2012; Celep et al. 2014; Kaya et al. 2015; Atalay et al. 2016). However, Donmez et al. (2012), Temel et al. (2015) and Acar & Satil (2019) reported that Stachys palustris L., Stachys byzantina C. Koch, Stachys bombycina Boiss., Stachys distans Bentham var. cilicica R. Bhattacharjee and Hub.-Mor. and Stachys distans Benth. var. distans does not contain sclerenchymatic tissue surrounding the vascular tissue in the anatomical structure of the stems. The sclerenchyma forms an unceasing ring-shaped tissue or separate bundles in the stem anatomy of Labiatae members. The sclerenchyma forms a separate bundles tissue in the stems of S. longiflora. These characters were reported to be used for separating sect. Ambleia species, especially close relatives (Dinc et al. 2008). Therefore, the sclerenchyma forms an unceasing ring-shaped tissue or separate bundles in the stem are useful characters for distinguishing the species.
Salmaki et al. (2011) reported that leaf anatomy provides valuable features that are useful in the classification of subgeneric classification as well as species delimitation in Stachys. These diagnostic characters have been described as the number of cell layers of palisade and spongy parenchyma, the shape of the transverse section, length of ventral/dorsiventral axis and type/thickness of collenchyma. Leaves of Lamiaceae members are generally dorsiventral or isobilateral (Metcalfe & Chalk 1950). This study showed that S. longiflora has a dorsiventral leaf. The same results have been also observed in the leaves of other Stachys taxa (Dinc & Ozturk 2008; Donmez et al. 2012; Temel et al. 2015; Kaya et al. 2015), but some Stachys specimens were observed to be isobilateral (Erdogan et al. 2012; Acar & Satıil 2019). According to the mesophyll structure, the leaves of S. longiflora have 1-3 layered palisade parenchyma under the upper epidermis and 2-3 layered spongy parenchyma under the palisade parenchyma. However, the leaves of Stachys balansae Boiss. & Kotschy ex Boiss. and Stachys carduchorum (R. Bhattacharjee) Rech.f. have 2 layered palisade parenchyma under the upper epidermis and 1 to 2 layered above the lower epidermis. In the center of mesophyll, there is 2 to 5 layered spongy parenchyma (Erdogan et al. 2012). Acar and Satil (2019) point out that leaves of Stachys aleurites Boiss. & Heldr. and S. distans var. distans has 2–3 layered palisade parenchyma under the upper epidermis and 4–5 layered spongy parenchyma under the palisade parenchyma those of S. bombycina has 1–2 layered palisade parenchyma under the upper epidermis and 3–4 layered spongy parenchyma under the palisade parenchyma, those of S. distans var. cilicica has 2 layered palisade parenchyma under the upper epidermis and 3–4 layered spongy parenchyma under the palisade parenchyma. Also, Kaya et al. (2015) reported that leaves of Stachys rupestris Bentham have 3–4 layered palisade parenchyma under the upper epidermis and 2–3 layered spongy parenchyma under the palisade parenchyma.
Moore et al. (1991) classified the pollen morphologies of Stachys sylvatica L., Stachys palustris L., Stachys arvensis (L.) L., Stachys annua (L.) L., Stachys germanica L., Stachys alpina L., Stachys recta L. and Stachys officinalis (L.) Trevisan under the group called Stachys sylvatica type. In this group, the pollen morphologies are trizonocolpate, pollen exines have reticulate ornamentation, and lumina are more or less uniform in size. Pollen morphologies of S. longiflora exhibit the features of S. sylvatica type as well. However, the pollen exine ornamentation is microreticulate in S. longiflora.
Salmaki et al. (2008) were examined nutlets of 31 taxa of Stachys, representing nine sections distributed in Iran, by Light and Scanning Electron Microscopy. Authors reported that, the basic shape of nutlets in most taxa is ovate, broad ovate, oval, triangular and oblong can also be found in few species. The nutlets of Stachys longiflora are elliptic-oblong and 2.89 mm–3.57 mm long and 1.27 mm × 1.43 mm wide, but the nutlet shapes of sect. Fragilicaulis species in Iran are ovate (Salmaki et al. 2008). The nutlet surface sculpturing pattern of Stachys taxa growing in Iran were studied and it was understood that these characters have a systematic value at the generic and specific levels (Salmaki et al. 2008). While the nutlets surface ornamentation of S. longiflora is a reticulate-papillate, sect. Fragilicaulis species in Iran are reticulate (Salmaki et al. 2008).
In the current study, trichomes were not found on the nutlets of any Stachys taxa (Salmaki et al. 2008; Satil et al. 2012). However, this study shows that the apex of the S. longiflora nutlets is long-haired which consists of 1-4 cells. This character is important for separating S. longiflora from the other members of sect. Fragilicaulis in Iran.
According to Bhattacharjee (1982), the flowering time of the species has been reported as of October. Field observations have shown that the species flowers from mid of June to early October. The altitude range belongs to S. longiflora has not been reported so far. Specimens were collected by the author only at an altitude of between 250 and 300 m. While the habitat of S. longiflora was reported in Flora of Turkey as “Mouth of limestone caves”, the specimens were collected from the limestone crevices.
Acknowledgments
The author is grateful to the curators of ANK, AEF, B, BM, E, G, GAZI, H, HUB, ISTE, ISTF, K and KNYA for permission to access their collections. I thank Dr. Muhittin Dinc for his support during the fieldwork. I am also grateful to both anonymous reviewers for their careful review of the manuscript. The author would like to thank the Necmettin Erbakan University Scientific Research Projects Coordination Unit under Grant No. 131210001 for the financial support.
References
Abu-Asab M.S., Cantino P.D. (1994). Systematic implications of pollen morphology in subfamilies Lamioideae and Pogostemonoideae (Labiatae). Annals of Missouri Botanical Garden 81: 653-686. https://doi.org/10.2307/2399915
Açar M., Satıl F. (2019). Comparative Micromorphological and anatomical investigations on the subsection distantes R. bhattacharjee (Stachys L./Lamiaceae). KSU J Agriculture Nature 22: 282–295.
Akcicek E. (2010). A new subspecies of Stachys cretica (section Eriostomum, Lamiaceae) from Turkey. Turk J Bot 34: 131-136. https://doi.org/10.3906/bot-0911-225
Akcicek E., Fırat M., Güner Ö. (2016). Stachys hakkariensis (Lamiaceae), a new species from eastern Anatolia (Turkey) belonging to Stachys sect. Olisia. Phytotaxa 257: 167-173. http://dx.doi.org/10.11646/phytotaxa.257.2.6
Atalay Z., Celep F., Bara F., Doğan M. (2016). Systematic significance of anatomy and trichome morphology in Lamium (Lamioideae; Lamiaceae). Flora 225: 60-75. https://doi.org/10.1016/j.flora.2016.10.006
Bhattacharjee R. (1974). Taxonomic studies in Stachys. I. New species and infra-sp ecific taxa from Turkey. Notes Royal Botanic Garden, Edinburgh 33: 275–292
Bhattacharjee R. (1980). Taxonomic Studies in Stachys II, A new infrageneric classification on Stachys L. Notes Royal Botanic Garden Edinburg 38: 65-96.
Bhattacharjee R. (1982). Stachys L. In: Davis, P.H. (Ed.) Flora of Turkey and East Aegean Islands. Edinburgh University Press. Edinburgh 7: 199-262.
Celep F., Kahraman A., Atalay Z., Doğan M. (2014). Morphology, anatomy, palynology, mericarp and trichome micromorphology of the rediscovered Turkish endemic Salvia quezelii (Lamiaceae) and their taxonomic implications. Plant Systematics Evolution 300: 1945-1958. https://link.springer.com/article/10.1007/s00606-014-1020-1
Celep F., Dirmenci T. (2017). Systematic and Biogeographic overview of Lamiaceae in Turkey. Natural Volatiles Essential Oils 4: 14-27. https://dergipark.org.tr/en/pub/nveo/issue/38934/454948
Davis P.H., Mill R.R., Tan K. (1988). Stachys L. In: Davis P.H., Mill, R.R., Tan, K. (Eds.) Flora of Turkey and the East Aegean Islands 10. Edinburgh University Press, Edinburgh pp: 199-264.
Dinç M., Doğan H.H. (2006). Stachys yildirimlii (Lamiaceae), a new species from south Anatolia, Turkey. In Annales Botanici Fennici pp: 143-147. https://www.jstor.org/stable/23727199
Dinç M., Öztürk M. (2008). Comparative morphological, anatomical, and palynological studies on the genus Stachys L. sect. Ambleia Bentham (Lamiaceae) species in Turkey. Turk J Botany 32: 113-121. https://journals.tubitak.gov.tr/botany/abstract.htm?id=9377
Dinç M., Doğu S. (2016). Stachys gaziantepensis (Lamiaceae), a new species from South Anatolia, Turkey. Proceedings of the National Acad Sci 86: 631-635. https://link.springer.com/article/10.1007/s40011-015-0511-3
Dirmenci T., Yıldız B., Akçiçek E., Martin E., Dündar E. (2011). Stachys vuralii (Lamiaceae), a new species from north Anatolia, Turkey. In Annales Botanici Fennici 48: 401-408. https://doi.org/10.5735/085.048.0503
Dönmez M., Kargıoğlu M., Temel M. (2012). The Morphological, Anatomical and Ecological Properties of Stachys palustris L. Afyon Kocatepe University J Sci Engineering 11: 1-9.
Duman H. (2000). Stachys L. In: Güner, A., Özhatay, N., Ekim, T., Başer, K.H.C. (Eds.) Flora of Turkey and the East Aegean Islands 11. Edinburgh University Press, Edinburgh pp: 204-206.
Ekim T., Koyuncu M., Vural M., Duman H., Aytaç Z., Adıgüzel N. (2000). Turkey Plants Red Data Book. Ankara, Turkey: Turkey’s Nature Protection Association pp: 246.
Akccedil E., Selvi S. (2012). Comparative anatomical studies on the two Stachys species (sect. Eriostomum, subsect. Germanicae) growing in Turkey. African J Pharmacy Pharmacol 6: 1417-1427. https://doi.org/10.5897/AJPP12.267
Govaerts R. (2019). World checklist of selected plant families. The Board of Trustees of the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/home.do
Harley R.M., Atkins S., Budantsev A., Cantino P.D., Conn B., Grayer R.J., Harley M.M, De Kok R., Krestovskaja T., Morales R., Paton A.J., Ryding O., Upson T. (2004). Labiatae. In: Kadereit, J. W. (Ed.) The families and genera of vascular plants 6. Springer pp: 241-242. IUCN (2014). Guidelines for using the IUCN Red List Categories and Criteria, version 11. IUCN Red List Unit http://www.iucnredlist.org/documents/RedListGuidelines
İlçim A., Çenet M., Dadandı M.Y. (2008). Stachys marashica (Lamiaceae), a new species from Turkey. In Annales Botanici Fennici 45: 151-155. https://doi.org/10.5735/085.045.0211
Kaya A., BAŞER K.H., SATIL F., Tümen G. (2000). Morphological and anatomical studies on Cyclotrichium origanifolium (Labill.) Manden. & Scheng.(Labiatae). Turk J Bot 24: 273-278. https://journals.tubitak.gov.tr/botany/issues/bot-00-24-5/bot-24-5-2-9909-8.pdf
Kahraman A., Celep F., Dogan M. (2009). Morphology, anatomy and palynology of Salvia indica L.(Labiatae). World Applied Sci J 6: 289-296. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.388.4990&rep=rep1&type=pdf
Kahraman A., Dogan M., Celep F., Akaydin G., Koyuncu M. (2010). Morphology, anatomy, palynology and nutlet micromorphology of the rediscovered Turkish endemic Salvia ballsiana (Lamiaceae) and their taxonomic implications. Nordic J Bot 28: 91-99. https://doi.org/10.1111/j.1756-1051.2009.00384.x
Kahraman A., Celep F., Doğan M. (2010). Morphology, anatomy, palynology and nutlet micromorphology of Salvia macrochlamys (Labiatae) in Turkey. Biologia 65: 219-227. Kahraman A., Celep F., Doğan M. (2010). Morphology, anatomy, palynology and nutlet micromorphology of Salvia macrochlamys (Labiatae) in Turkey. Biologia 65: 219-227. https://link.springer.com/article/10.2478/s11756-010-0013-y
Kaya B., Dinç M., Doğu S. (2015). AnAtoMIcAl chAracterIstIcs of turkIsh endeMIc StachyS rupeStrıS Montbret et Aucher ex benthAM (lAMIAceAe). Modern Phytomorphol 8: 37-40. https://www.phytomorphology.com/articles/anatomical-characteristics-of-turkish-endemic-stachys-rupestrs-montbret-et-aucher-ex-bentham-lamiaceae.pdf
Meidner H., Mansfield T.A. (1968). Physiology of stomata. Physiology of stomata pp: 138-141. https://www.cabdirect.org/cabdirect/abstract/19701701808
Metcalfe C.R., Chalk L. (1950). Anatomy of the Dicotyledons. Oxford University Press 1: 810-816.
Moore P.D., Webb J.A., Collinson M.E. (1991). Pollen Analysis. Second edition, Oxford: Blackwell Scientific Publications pp: 1-216.
Özhatay F. N., Kültür Ş., Gürdal M.B. (2011). Check-list of additional taxa to the supplement Flora of Turkey V. Turk J Bot 35: 589-624. https://journals.tubitak.gov.tr/botany/issues/bot-11-35-5/bot-35-5-10-1101-20.pdf
Punt W., Hoen P.P., Blackmore S., Nilsson S., Le Thomas A. (2007). Glossary of pollen and spore terminology. Rev Palaeobotany Palynology 143: 1-81. https://doi.org/10.1016/j.revpalbo.2006.06.008
Rechinger K.H. (1982). Stachys L. In: Rechinger, K.H. (Ed.) Flora Iranica Akademische Druck-und Verlagsanstalt. Graz 150: 354-396.
Salmaki Y., Zarre S., Jamzad Z. (2008). Nutlet micromorphology and its systematic implication in Stachys L.(Lamiaceae) in Iran. Feddes Repertorium 119: 607-621.
Salmaki Y., Zarre S., Lindqvist C., Heubl G., Bräuchler, C. (2011). Comparative leaf anatomy of Stachys (Lamiaceae: Lamioideae) in Iran with a discussion on its subgeneric classification. Plant Systematics and Evolution 294: 109-125. https://link.springer.com/article/10.1007/s00606-011-0450-2
Satıl F., Kaya A., Akçiçek E., Dirmenci T. (2012). Nutlet micromorphology of Turkish Stachys sect. Eriostomum (Lamiaceae) and its systematic implications. Nordic J Bot 30: 352-364. https://doi.org/10.1111/j.1756-1051.2011.01306.x
Temel M., Kargıoğlu M., Arı S. (2015). The morphological, anatomical and ecological features of Stachys byzantina (Lamiaceae) naturally distributed in Afyonkarahisar. Süleyman Demirel Üniversitesi Fen Edebiyat Fakültesi Fen Dergisi 10: 35-47.
Uysal I., Ozturk M., Pirdal P. (1991). Morphology, anatomy and ecology of endemic species, Sideritis trojana Bornm. Doga-Tr. J. of Botany 15: 371-379.
Uysal İ. (2002). Stachys cretica L. subsp. Smyrnaea Rech Elephant. Studies on the morphology, anatomy and ecology of the endemic taxon. Ecol Environ J 11: 16-20. https://app.trdizin.gov.tr/publication/paper/detail/TWpnMU56WTI= Uysal İ. (2003). Studies on the morphology, anatomy and ecology of Stachys thirkei C. Koch (Oregano). J Herb Systematic Bot 10: 129-141. https://app.trdizin.gov.tr/publication/paper/detail/TXpBeU5qUTA
Vardar Y. (1987). Preparation Technique in Botany. Ege University Faculty of Science Printing House pp: 25-26.
Yildirimli S. (2010). Some new taxa, records and taxonomic treatments from Turkey. Herb J Syst Bot 17: 1-114. http://media.e-taxonomy.eu/cichorieae/protolog/pdf/Lactuca_kemalya.pdf
Yılmaz Ö. Daşkın R., Kaynak G. (2010). Stachys pseudobombycina sp. nov. (Lamiaceae) from south Anatolia. Nordic J Bot 28: 341-343.
Wodehouse R.P. (1935). Pollen Grains. McGraw-Hili Book Co. Inc., NewYork. pp: 67-84.