Research - Modern Phytomorphology ( 2021) Volume 15, Issue 1
An efficient plant regeneration, detection and identification of secondary metabolites from propagate plants of Peperomia pellucida (L.) for mass cultivatione
Abdul Bakrudeen Ali Ahmed1,2,3, Teoh Lydia1, Muneeb Muhamed Musthafa4, Rosna Mat Taha1 and Faiz MMT Marikar52Department of Biochemistry and Biotechnology, Center for Research and Development (CRD), PRIST University, Vallam, Thanjavur - 613403, Tamil Nadu, India
3Department of Applied Sciences, Ton Duc Thang University, Ho Chi Minh City, Vietnam
4Department of Biosystems Technology, South Eastern University of Sri Lanka, University Park, Oluvil, 32360, Sri Lanka
5Department of Health Science and Education, The Open University of Sri Lanka, 10350, Sri Lanka
Received: 23-Nov-2020 Accepted: 03-Dec-2020 Published: 11-Dec-2020, DOI: 10.5281/zenodo.5078526
Abstract
Several species in the genus Peperomia Ruiz and Pav have giant chloroplasts in the palisade parenchyma of their leaves. Because of this unique feature, Peperomia pellucida L. has become a valuable model plant for studying plastid biology. However, the use of Peperomia for molecular studies has been limited by the lack of efficient regeneration and transformation protocols. This study describes an effective protocol for in vitro shoot induction, plant regeneration and rooting from internode of explant of Peperomia pellucida using Murashige and Skoog (MS) medium supplemented with different plant growth regulators. The multiple shoots were induced from internodes cultured on MS medium containing Kinetin (KN) (0.5 mg L-1) alone which induced six shoots per internodal explant. In combination Benzyladenine (BA) (1.5 mg L-1) and KN (1.5 mg L-1) induced 10 shoots per explant, while, length of shoots were significantly increased in BA (1.0 mg L-1) and KN (3.0 mg L-1) (0.8 cm per explant); KN (0.5 mg L-1) (0.7 cm per explant) respectively. Adventitious root induction was achieved on MS media with different concentrations of Indole-3-Butricacide (IBA) and Indole-3-Acetic Acid (IAA). The successful root induction was observed in MS medium supplemented with IBA and shown 100% responses while plantlets survival was 90% at greenhouse garden soil. Rooted plantlets were successfully acclimatized to pot containing soil, sand and farm manure (1:1:1) and established in tissue culture room. In vitro methanol and ethanol extracts gave the highest amount of Apiol and Phytol content in GC-MS analysis. This protocol could be very useful for mass cultivation of Peperomia pellucida for industrial exploitation with selected and standardized plants productiont.
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
blog
Keywords
Regeneration, root, shoot, metabolites, micropropagation, growth regulator
Introduction
Genus Peperomia is one of the largest genera of basal angiosperms, comprising about 1500-1700 species belongs to the family Piperaceae (Ilyas et al. 2014). Peperomia pellucida is a common fleshy tropical annual fibrous shallow-rooted herb, which grows as succulent and erect plant (Majumder 2012). This plant grows in moist habitat (Ooi et al. 2012) and can reach up to 15-45 cm long. The stem part is fleshy, round, delicate and glabrous (Majumder 2012) whereas the seeds are tiny dot like and attached to several fruiting spikes. The leaves are arranged alternately on the stalks for about 1-2 cm long. It has shiny heart-shaped fleshy leaves and the blades of the leaves are broadly-ovate. The flowers are numerous, scattered on the spike and without petals but with only stamens and an ovary. It also has fruits which are minute, and seeds produce mustard-like odour when crushed (Majumder 2012). Now this plant is pantropical in distribution and is abundantly available in Malaysia. This plant has been reported from Bangladesh, Philippines, Thailand, Vietnam, Indonesia, Spain and Japan (Khan et al. 2010; Mutee et al. 2010).
P. pellucida served as valuable components of food, medicines and has played a significant role in improving the quality of human life. Majumder et al., (2011) and Sheikh et al., (2013) reported that the leaves and stems of P. pellucida may be eaten as vegetable. The fresh plant has the crispness celery and carrot stick as in salads. This plant is used for treating abdominal pain, boils, colic fatigue, abscesses, acne, convulsions, fever, gout, headache, renal disorders, to lower blood cholesterol level, conjunctivitis, skin diseases, breast cancer and rheumatic joint pain (Khan et al. 2010; Ooi et al. 2012). To cure haemorrhage, the whole plant was crushed and mixed with water to form a mixture; then heated and taken orally (Majumder et al. 2011). The aerial parts are extracted and used as dressing for wounds curing and their roots are used to treat fevers. P. pellucida also has a popular emollient, cough suppressant and diuretic in Guyana and the Amazon as well as effective in the treatment of proteinuria. Aerial parts of P. pellucida demonstrated dose-dependent analgesic and anti-inflammatory actions by interfering with prostaglandin synthesis (Mutee et al. 2010).
Since, plants are used in modern medicine as sources of direct therapeutic agents of many diseases. More complex semisynthetic chemical compounds, used as a raw base material (Borokini and Omotayo 2012). Khan et al. (2010) revealed, the presence of flavonoids, apiols, phytosterols, substituted styrenes, secolignans, tetrahydrofuranlignans, highly metaoxylateddihydronaphthalenone, arylpropanoiids, sesamin, isoswertisin, xanthone glycoside and peperomins A, B, C and E. The flavonoids and phytosterols such as acacetin, apigenin, arylpropanoids (e.g. apiols), isovetexin, pellucidatincampesterol and stigmasterol, substituted styrenes and dimeric ArC2 compound or pellucidin A have been isolated from P. pellucida (Majumder 2011). Whereas the compound of phytosterolscampesterol Acacetin, apiigenin, isovitexin and pellucidatin and stigmasterol (Majumder 2011). Since this plant has more pharmacological application, there is a requirement to establish an effective and efficient propagation protocol for production of bioactive compounds and sustainable conservation to the pharmaceutical industries. Plant tissue culture offers a viable alternative tool, widely used for conservation, commercial propagation and bioactive compound production. This in vitro technique is very useful in ensuring sustainable optimization sources of plant derived natural products. In addition, this propagation method enhances optimal extraction of secondary metabolites by use of elicitors and applicable for large scale products. The main aims of the current study are to establish highly efficient regeneration system for this species and to determine the secondary metabolites content in dry in vivo leaves.
Materials and Methods
Plant collection and identification
Peperomia pellucida were collected from a population growing at the Institute of Biological Science garden of University Malaya and from various part of University Malaya (Fig. 1a). The voucher specimens were verified (No. KLU47794) (Fig. 1b) at the University of Malaya Herbarium (KLU), Kuala Lumpur, Malaysia with the voucher of specimen.

Figure 1: (a) Natural habitat; (b) Herbarium seed; (c) Shoots response in Murashige and Skoog (MS) medium; (d) Shoots response in (MS) supplemented with Butricacide (BA) 1.5 mg L-1; (e) Shoots response in MS supplemented with Kinetin (KN) 0.5 mg L-1; (f) Shoots response in MS supplemented with KN 1.0 mg L-1; (g) Shoots response in MS supplemented with BA 1.0 mg L-1+KN 2.5 mg L-1; (h) Shoots response in MS supplemented with BA 1.5 mg L-1+1.5 mg L-1 KN. Shows root response in (i) Indole-3-Acetic Acid (IAA) 1.0; (j) IAA 2.0; (k) Indole-3- Butricacide (IBA) 0.5; (l) IBA 1.0; (m) IBA 1.5; (n) IBA 2.0.
Surface sterilization
The plants were washed under running tap water for 30 min. and aerial parts of the plants were soaked and washed with three (3) drops of teepol and rinsed with sterile distilled water for 5 times. Then washed with 50% sodium hypochlorite (v/v) for 2 min. and followed by washing in a sterile 0.1% mercury chloride (w/v) for 1 min and rinsing with sterile distilled water for 5 times. Finally, explants were treated with an antifungal, 0.1% carbendazime (w/v) diluted in 70% alcohol (v/v) for 30 sec and rinsed with sterile distilled water for 5 times.
Shoot regeneration and rooting
The surface sterilized leaves and roots explants were removed from the shoots explants (1-1.5 cm) of P. pellucida. The internodal explants were cultured on MS medium (Murashige and Skoog medium) supplemented with Cytokinins, BA (Benzyl Adenine) (0.5-2.0 mg L-1); KN (Kinetin) (0.5-3.0 mg L-1); Auxin, IAA (Indole Acetic Acid) (0.5-2.0 mg L-1); IBA (Indole Butyric Acid) (0.5-2.0 mg L-1) respectively. The pH of the media was adjusted to 5.7-5.8 and Carbendazime (10 mg L-1) was added into the medium in order to prevent the fungal contamination. Finally, the prepared medium was autoclaved at 121°C for 20 min. After autoclaved, the medium was dispensed in a culture tubes in laminar flow. The photoperiod of light was maintained for 16 h/8 h light/darkness. The culture room usually maintained at 25 ± 2°C. Humidity of the culture room also must be controlled at 75–90% and uniform forced-air ventilation. The healthy multiple shoots formed were transferred into rooting medium supplemented with IBA (0.5-2.0 mg L-1) and IAA (0.5-2.0 mg L-1) concentration.
Acclimatization
All plantlets were taken out carefully from the culture containers after 6 weeks and washed to remove the agar. The plantlets were transferred to plastic pots (5 cm diameter) containing soil, sand and farm manure (1:1:1) and maintained at 25± 2°C, 16 h day length (35-50 M Fm-2 S-2) and at 75-80% relative humidity. The grown plantlets were transplanted into clean pots (10 cm diameter) containing natural soil, kept under shade conditions for 2 weeks and finally moved to the University of Malaya green house.
Extraction and phytochemical analysis of secondary metabolites
The leaves of P. pellucida were collected and dried under the shadow conditions and ground it up to the powder, where 10 g of the dry powdered was placed into thimble. Then, the thimble inserted into soxhlet apparatus and extracted with 300 ml ethanol for 48 h. After complete extraction, the solvent was evaporated and concentrated to dry residue. Afterwards, the concentrated residue was treated to a non-polar solvent to detect the presence of alkaloid. The concentrated dry residue (0.01 g) was diluted in 5 ml of distilled water and the pH was adjusted to 7.00 ± 0.05. This mixture residue was diluted with ethyl acetate (5 ml) for 4 times and these dilutions were placed on petri disc. The combined extracts were evaporated and treated with 0.3 ml of Bis (trimethylsilyl) acetamide (BSA) reagent up to the complete removal of extract. The volume was adjusted to 1 ml with BSA and gently shacked for 20 min. Finally, the treated extraction was diluted by using 0.1 ml treated extraction with 0.9 mL of BSA.
Gas Chromatography- Mass Spectra (GC-MS)
The phytochemical properties of treated extraction were determined by using Agilent gas chromatography-mass spectrometry (GC-MS) equipped with HP-5MS column (30 m x 0.25 mm i.d., 0.25 µm film thickness, J&W Scientific Inc, USA). The GC-MS were run by using the following method. The initial temperature was held at 40°C for 2 min, and increased at 3°C/min to 140°C/min, then ramped from 140°C to 250°C at the rate of 10°C/min. The carrier gas was helium at the flow rate of 1 mL min-1. The injection temperature was maintained at 250°C. The samples (1 µL) were injected separately, with a split ratio of 1:10. The mass spectra were recorded over the range 50-650 amu at one scan per second, with ionization energy of 70 eV and ion source temperature at 230°C. Extraction was done in a methanol method.
Statistical analysis
Statistical analysis was done using SPSS 14.0 for windows integrated student version. All the experiments were repeated twice and used replicates. The effect of different treatment was quantified as mean ± SE and the data were subjected to statistical analysis using DMRT (Duncan’s Multiple Range Test) at 5% level.
Results
An efficient surface sterilization and mass propagation method was successfully accomplished in this technique for P. pellucida. The P. pellucida internodal explants were used for mass propagation and showed 90% of bacterial and fungal contamination. In order to avoid the contamination, pre-treatment of the explants were tried in surface sterilization using carbendazime (10 mgL-1) (0.01% of Carbendazime in 70% alcohol) used in medium, which resulted in healthy internodal explants throughout the experiment. Further, the survival rate of acclimatized plantlets was 90% and they exhibited the identical morphological characteristics of the mother plants.
Shoot regeneration and rooting
As Fig. 1 illustrates the percentage of culture response and the number of shoot induction observed in the presence of Cytokinins. The internodal explants were also cultured on MS basal (MSO) as control. MSO showed (Fig. 1) the highest percentage (96.7%) of response compared to MS medium supplemented with PGRs (Plant Growth Regulator). Different PGRs responses were observed in MS supplemented with BA 0.5 mgL-1 (90%) and MS with KN 1.0 mgL-1 (93%). The highest percentage of response was observed in three combinations, such as BA (1.0 mg L-1) with KN (0.5 mg/L); BA (1.0 mg L-1) with KN (2.0 mg L-1) and BA (1.5 mg L-1) with KN (1.5 mg L-1). Overall, the lowest percentage of response was observed in (BA 1.0 mg L-1) with KN (1.5 mg L-1) (Tab. 1).
| Plant growth regulators (mg L-1) |
Percentage of response (%) | Root biomass/explant Mean ± SE |
| MS+IBA 0.5 | 85 | 0.90 ± 0.06c |
| MS+IBA 1.0 | 90 | 0.97 ± 0.03ab |
| MS+IBA1.5 | 90 | 0.97 ± 0.03ab |
| MS+IBA2.0 | 98 | 1.00 ± 0.00a |
| MS+IAA0.5 | 0 | 0.00 ± 0.00f |
| MS+IAA1.0 | 0 | 0.00 ± 0.00f |
| MS+IAA1.5 | 5 | 0.07 ± 0.05de |
| MS+IAA2.0 | 10 | 0.10 ± 0.06d |
Table 1. Percentage of root response using Murashige and Skoog (MS) media with Indole-3- Butricacide (IBA) and Indole-3-Acetic Acid (IAA), at 6th weeks of culture. [Mean followed by the different letters in each column is significantly different at the P < 0.05 level means were compared using Duncan’s Multiple Range Test (DMRT)].
proliferation compared with other explants. P. pellucida internodal explants were cultured on MS medium containing different concentration of PGRs of BA (0.5–2.0 mg L-1) and KN (0.5-2.0 mg L-1) for shoot multiplication and shoot elongation (Fig. 2 and 3). The means of shoot length against the different PGRs on internodal explant of P. pellucida is shown in Fig. 4. All concentration of BA and KN promoted multiplication after 14 days of culture. Adventitious shoots were induced from internodal explants cultured on the MS medium supplemented with BA (0.5–2.0 mg L-1) and KN (0.5-3.0 mg L-1), where all the concentrations showed 70% responses. The highest number of shoots was achieved in KN (0.5 mg L-1) with 6 shoots per explant (Fig. 2). For the MS medium with BA (1.0 mg L-1) alone, showed 3 shoots per explant. MS medium supplemented with different combination and concentration of BA and KN also managed to produce shoots. When BA combined with KN, a variety of responses were observed: equal combination of BA (1.5 mg L-1) and KN (1.5 mg L-1) gave the highest shoot initiation. The equivalent concentration of BA with KN (10 shoots per explant) is better than BA alone and KN alone for shoot regeneration of P. pellucida.
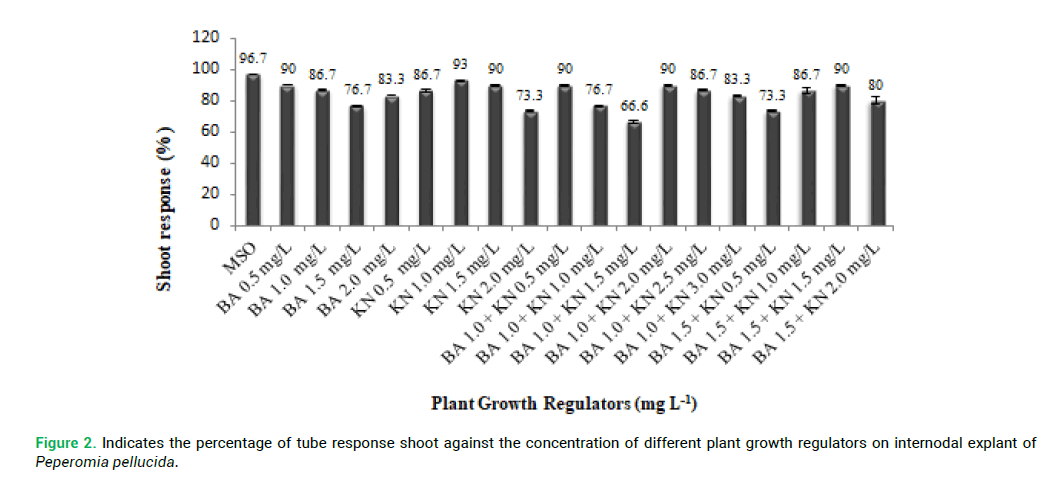
Figure 2: Indicates the percentage of tube response shoot against the concentration of different plant growth regulators on internodal explant of Peperomia pellucida.
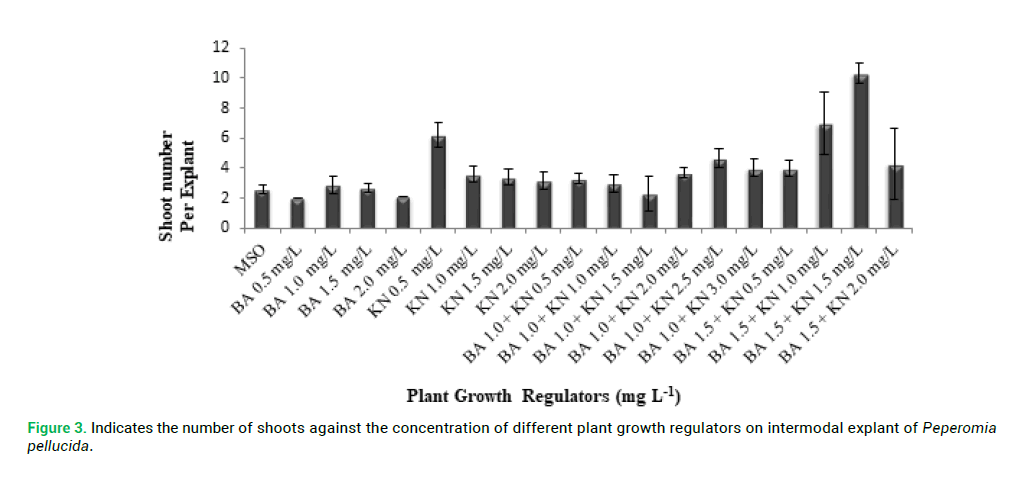
Figure 3: Indicates the number of shoots against the concentration of different plant growth regulators on intermodal explant of Peperomia pellucida.
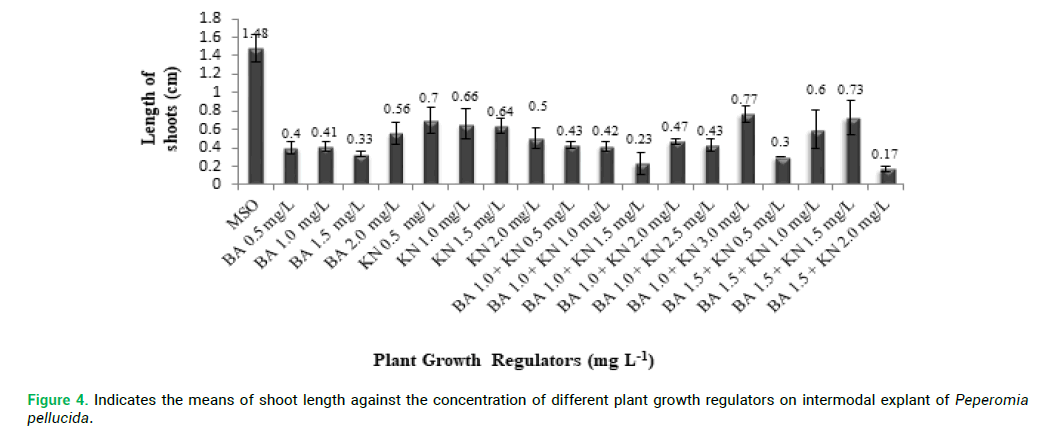
Figure 4: Indicates the means of shoot length against the concentration of different plant growth regulators on intermodal explant of Peperomia pellucida.
Based on Fig. 4, all concentration of BA and KN promoted the multiple shoots in internodal explants of P. pellucida. After 6 weeks of culture, shoot length was significantly decreased than BA with KN. The shoot elongation of internodal explant cultured on MS medium without PGRs showed shoot length of 1.48 cm per explant. The shoot length was significantly increased in KN (0.5 mg L-1) (7.0 cm). For the MS medium with BA alone, the shoot length was shortened at BA (2.0 mg L-1) (0.56 cm per explant) whereas, kinetin alone, MS with KN 0.5 mg L-1) the shoot length was (0.7 cm per explant). On the other hand, MS medium supplemented with different combination and concentration of BA and KN gave increased shoot length than BA and KN alone. BA (1.0 mg L-1) combined with KN (3.0 mg L-1), showed higher shoots length (7.7 cm per explant). Combination of BA (1.5 mg L-1) and KN (2.0 mg L-1) displayed the lowest shoot length (1.7 cm per explant).
After week 6, the regenerated shoots were separated and transferred to MS medium supplemented with IBA (0.5-2.0 mg L-1) and IAA (0.5-2.0 mg L-1). Auxins did not cause any decay process on the shoots. After two (2) weeks of culturing, the adventitious shoots in IBA started to from roots. The response of roots in MS medium supplemented with IBA and IAA is displayed in Fig. 4 and the percentage of growth is shown in Tab. 1. The root induction was achieved in IBA at 2.0 mg L-1 (98%) followed by IBA at 1.0 mg L-1 and 1.5 mg L-1 (90%), while, IAA at 0.5 mg L-1 and IAA at 1.0 mg L-1 did not induce any roots.
Since there is no tissue culture and propagation method has been reported on P. pellucida plant (Loc et al. 2010), this study is a big step forward due to the numerous medicinal advantages of this herb. There are few studies conducted on Genus Peperomia for propagation but none of them specifically tested for P. pellucida. Naggar and Osman (2014) reported micropropagation of Peperomia obtusifolia using BA and KN media where KN (5 mg L-1) combined with α-Naphthalene Acetic Acid (NAA) (1 mg L-1) displayed the most effective concentrations for shoot proliferation, number of shoots, fresh weight, total leaf area, total chlorophyll and callus weight. For P. obtusifolia KN was inefficient compared to Benzylaminopurine (BAP) but MS media supplemented with the later resulted in multiple adventitious root development (Al-Ahmad 2015). Rana et al. (2015) reported shoot bud induction and shoot proliferation was achieved in the presence of Cytokinin, BA or KN alone whereas, shoot regeneration was achieved in combination of BA (1.5 mg L-1) with KN (1.5 mg L-1) with 10 shoots per explant in vitro from this study. Moreover, the highest shoot length was achieved in MS medium without hormone with 1.48 cm per explant. The explants with PGRs produced shoot taller than MS medium without PGRs.
The maximum length of shoot in MS medium supplemented with BA (1.0 mg L-1) with KN (3.0 mg L-1). Al-Ahmad (2015) reported, IBA was used for in vitro rooting of Peperomia obtusifolia, while, the concentration of IAA at 1.5 mg L-1 (95%) and IAA at 2.0 mg L-1 induced some roots (10%) when maintained under long days. IBA was suitable for rooting and successful plantlet formation of P. pellucida due to its stability against catabolism and inactivation by conjugation (Koetle et al. 2010). Peperomia species regeneration using MS media supplemented by Gibberellic acid responded with some promising results in vitro via organogenesis, where, the regeneration tests were conducted using P. peduncularis and P. metallica leaf segments displayed positive responses (Ahmadabadi and Bock 2010). Nevertheless, responses of hormones such as Eatin, Thidiazuron (TDZ), and Gibberellic acid have been reported to show some significant impact on shoot initiation which is yet to be examined extensively (Lincy and Sasikumar 2010; Sahai et al. 2010). Auxins play a vital part in regeneration of plant tissues in vitro culture, which has been reiterated on this study as well, but it does not act alone. Auxins act rather in conjugation with other plant regulators in inducing growth and development (Sauer et al. 2013). In addition, Cytokinins also show some significant involvement in plant cell division in cell and tissue cultures.
Extraction and phytochemical analysis of secondary metabolites using GC-MS
The GC-MS analysis for phytocomponents identification via methanol and ethanol extract of the leaves of Peperomia pellucida were shown Tab. 2 and 3 respectively. Through GC-MS analysis, we determined the major component of alkaloids. The major constituents found in the methanol and ethanol extracts were apiol and followed by phytol. There other constituents resulted from the extracts were also presented on Tab.2 and 3.
| Constituents | Retention Time | Area sum (%) |
| Apiol | 8.226 | 59.95 |
| Phytol | 10.764 | 6.68 |
| Spathulenol | 8.06 | 4.5 |
| Silane | 15.17 | 3.86 |
| 1,6,10-Dodecatrien-3-ol | 10.374 | 3.36 |
| 1,5-Cyclodecadiene | 10.72 | 2.56 |
| Octadecanoic acid | 9.835 | 2.04 |
| 6-Octen-1-ol | 9.605 | 1.87 |
| Thiocyanic acid, 4-oxotricyclo[3.3.1.1(3,7)]dec-2-yl ester, (1.alpha.,2.beta.,3.beta.,5.alpha.,7.beta.) | 8.547 | 1.78 |
| 6-Octen-1-ol | 9.502 | 1.6 |
| p-Mentha-[1(7),8]-diene | 8.119 | 1.41 |
| 1,3-Bis-(2-cyclopropyl,2-methylcyclopropyl)-but-2-en-1-one | 8.942 | 1.18 |
Table 2. Phytocomponents identified in the methanol extract of the leaves of Peperomia pellucida by GC-MS.
| Constituents | Retention Time | Area Sum (%) |
| Apiol | 8.222 | 37.17 |
| Phytol | 10.758 | 12.59 |
| Heptadecane | 16.713 | 5.33 |
| Hexadecanoic acid methyl ester | 9.831 | 4.99 |
| Linolenic acid | 10.716 | 4.33 |
| Bicyclo[7.2.0]undec-4-ene,4,11,11-trimethyl-8-methylene | 7.019 | 3.07 |
| Spathulenol | 8.056 | 2.02 |
| 6-Octen-1-ol, 3,7-dimethyl-, propanoate | 9.601 | 1.73 |
| 1-Iodo-2-methylnonane | 8.603 | 1.71 |
| 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, [S-(Z)- | 10.37 | 1.32 |
| 1H-3a,7-Methanoazulene, octahydro-1,4,9,9-tetramethyl- | 8.55 | 1.23 |
Table 3. Phytocomponents identified in the methanol extract of the leaves of Peperomia pellucida by GC-MS.
There has been growing interest in alternative therapies, especially the use of natural compounds derived from plants. The presence of Apiol and Phytol in this plant extracts are pharmacologically useful as an astringent, where antifungal activity has been reported for the former, which was also reported by Verma et al. (2015). Phytol, being the important compound in P. pellucida is an important diterpenes and possessed antibacterial and anticancer activities (Wei et al. 2011; Lee et al. 2016). Egwuche et al. (2011) reported alkaloids are the most efficient therapeutically significant phytochemical derivative. Methanol extract of P. pellucida leaf resulted in the presence of Hexadecanoic acid, methyl ester and Octadecadienoic acid, which possesses antioxidant and anticancer property (Wei et al. 2011). It was revealed the presence of Spathulenol in the extract of P. galioides and P. chalhuapuquiana. Peperomia tetraphylla has been used for the extraction of Amides and six (6) amides were isolated and identified for the first time in 2010 (Li et al. 2010). P. tetraphylla was also reported to contain a novel Norlignan and a novel Phenylpropanoid from the ethanol extracts of the whole plant. Further work needs to be done in the future to correlate the specific compound with their respective standard. It could have potential as a pharmaceutical drug in future.
It is clear that the plant regeneration, detection and identification of secondary metabolites from propagate plants of P. pellucida mass cultivation had an effect on different level of anticancer, antimicrobial and antioxidant properties, which of importance for the development of new therapeutic agents. Further works need to be done in the future to correlate the specific compound with its biological property.
Conclusion
In conclusion, the internodal explant of P. pellucida showed successful plant regeneration with an efficient micropropagation protocol. BA, KN alone and in their combinations induced the shoots and subsequent proliferation. The number of shoots formed was varied based on the presence of PGRs. This was attributed to different plant species have different sensitivity towards the number and length of regenerated shoots. IBA was the most suitable PGR for root induction of P. pellucida. Plantlets were successfully acclimatized in the field. The cytological studies revealed that, there were 2n = 38 chromosomes in in vivo and 2n = 36, in in vitro plant from 20 cells observed. This may be due to somaclonal variation that occurs in the chromosome number of the plant although not significant. However, the precise mechanism as to how these factors affect chromosome numbers remains to be determined. GC-MS analysis was used to determine the components of alkaloids in Peperomia pellucida.
Acknowledgements
The authors would like to thank University of Malaya Research Grant (RG078-12B10) and (RP025-2012B).
Authors' Contributions
Abdul Bakrudeen Ali Ahmed and Rosna Mat Taha designed the research. Teoh Lydia carried out most of the laboratory works and Muneeb M. Musthafa and Faiz MMT Marikar contributed in writing the manuscript and analysis the results.
References
Ahmadabadi M., Bock, R. (2010). Development of a highly responsive leaf-based regeneration system for Peperomia species. Turk J Bot 34: 329-335. https://doi.org/10.3906/BOT-1001-309
Al-Ahmad H. (2015). Differential response of leaf and stem explants to growth regulators and direct organogenesis of baby rubber plant (Peperomia obtusifolia). Adv Health Sci Educ 2: 68-78. https://staff.najah.edu/media/published_research/2016/05/08/V2N1-007.pdf
Borokini T.I. and Omotayo F.O. (2012). Comparative phytochemical analysis of selected medicinal plants in Nigeria. IJAR 1: 11-18.
Egwuche R.U., Detola O.A.A., Erukainure O.L. (2011). Preliminary investigation into the chemical properties of Peperomia pellucida L. Res J Phytochem 5: 48-53. http://doi.org/10.3923/rjphyto.2011.48.53
Ilyas S., Naz S., Aslam F., Parveen Z., Ali, A. (2014). Chemical composition of essential oil from in vitro grown Peperomia obtusifolia through GC-MS. Pak J Bot 46: 667-672.
Khan A., Rahman M. and Islam M.S. (2010). Isolation and bioactivity of a xanthone glycoside from Peperomia pellucida. LSMR 1: 1-9. https://astonjournals.com/manuscripts/Vol2010/LSMR-1_Vol2010.pdf
Koetle M.J., Finnie J.F., Van Staden J. (2010). In vitro regeneration in Dieramaerectum Hilliard. Plant Cell Tissue 103: 23-31. https://doi.org/10.17660/ActaHortic.2008.792.46
Lee S.W., Sim K.Y., Wendy W., Zulhisyam A.K. 2016. Peperomia pellucida leaf extract as immunostimulator in controlling motile aeromonad septicemia due to Aeromonas hydrophila in red hybrid tilapia, Oreochromis spp. farming. Vet World 9: 231-234. https://doi.org/10.14202/vetworld.2016.231-234
Li Y., Gong Z., Ma C., Feng X., Huang J. (2010). Amides from Peperomia tetraphylla. China J Chinese Material Medica 4: 1-6.
Lincy A., Sasikumar B. (2010). Enhanced adventitious shoot regeneration from aerial stem explants of ginger using TDZ and its histological studies. Turk J Bot 34: 21-29. https://doi.org/10.3906/bot-0805-6
Loc N.H., Bach N.H., Kim T., Yang M. (2010). Tissue culture and expression of Escherichia coli heat-labile enterotoxin B subunit in trangenic Peperomia pellucida. Protein Expr Purif 72: 82-86. https://doi.org/10.1016/j.pep.2010.02.010
Majumder P. (2011). Phytochemical, pharmacologist and physicochemical standardization of Peperomia pellucia (L.) HBK. Stem. Pharmacie Globale 2: 1-4.
Majumder P. (2012). Evaluation of taxo-chemical standardization and quality control parameters of Peperomia pellucida (Family: Piperaceae): A multi valuable medicinal herb. J Pharm Sci Innov 1: 7-12. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Mutee A.F., Salhimi S.M., Yam M.F., Lim C.P., Abdullah G.Z., Ameer O.Z., Abdulkarim M.F., Asmawi M.Z. (2010). In vivo anti-inflammatory and in vitro antioxidant activities of Peperomia pellucida. Int J Pharmacol 6: 686-690. https://doi.org/10.3923/ijp.2010.686.690
Naggar H.M.E., Osman A.R. (2014). Micropropagation and organogenesis of Peperomia obtusifolia. Asian J Crop Sci 6: 58-66. https://doi.org/10.3923/ajcs.2014.58.66
Ooi D.J., Iqbal S., Ismail M. (2012). Proximate composition, nutritional attributes and mineral composition of Peperomia pellucida L. (Ketumpangan Air) grown in Malaysia. Molecules 17: 11139-11145. https://doi.org/10.3390/molecules170911139
Rana S.K., Oli P.S., Rana H.K. (2015). Traditional botanical knowledge (TBK) on the use of medicinal plants in Sikles area, Nepal. Asian J Plant Sci 5: 8-15. https://www.imedpub.com/articles/traditional-botanical-knowledge-tbk-on-the-use-of-medicinal-plants-in-sikles-area-nepal.pdf
Sahai A., Shahzad A., Anis M. (2010). High frequency plant production via shoot organogenesis and somatic embryogenesis from callus in Tylophora indica, an endangered plant species. Turk J Bot 34: 11-20. https://doi.org/10.3906/bot-0809-3
Sauer M., Robert S., Kleine-Vehn J. (2013). Auxin: simply complicated. J Exp Bot 64: 2565-2577. https://doi.org/10.1093/jxb/ert139
Sheikh H., Sikder S., Paul S. K., Hasan A.M.R., Rahaman M.M., Kundu S.P. (2013). Hypoglycemia, anti-inflammatory and analgesic activity of Peperomea pellucida (L.) HBK (Piperaceae). Int J Pharm Sci 4: 458-463. http://dx.doi.org/10.13040/IJPSR.0975-8232.4(1).458-63
Verma R.S., Rajendra C.P., Prakash G., Amit C. (2015). Essential oil composition of Peperomia pellucida (L.) Kunth from India. J Essent Oil Res 27: 89-95. https://doi.org/10.1080/10412905.2014.982878
Wei L.S., Wee W., Siong J.Y.Y., Syamsumir D.F. (2011). Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Medica Iranica 49: 670-674. https://acta.tums.ac.ir/index.php/acta/article/view/3816/3791