Research - Modern Phytomorphology ( 2022) Volume 16, Issue 1
Agronomical responses of wheat cultivars to deficient water stress
Keyvan Shams*Keyvan Shams, Department of Crop Production, College of Agriculture, Kermanshah Branch, Islamic Azad University, Iran, Email: keyvan@iauksh.ac.ir
Received: 17-Jan-2022, Manuscript No. mp-22-51764 ; Accepted: 27-Jan-2022, Pre QC No. mp-22-51764 (PQ); Editor assigned: 19-Jan-2022, Pre QC No. mp-22-51764 (PQ); Reviewed: 24-Jan-2022, QC No. mp-22-51764 (Q); Revised: 25-Jan-2022, Manuscript No. mp-22-51764 (R); Published: 04-Feb-2022, DOI: 10.5281/zenodo.7731554
Abstract
Two similar and concurrent experiments were carried out in the Agricultural Research Center of Islamic Azad University, Kermanshah Branch and Agricultural Research Center of Islamabad during 2016-2017. The experiments were performed in a split-plot format in a randomized complete-block design based on 3 replications. The main plots were assigned to 4 different regimes of irrigation: (I1) full irrigation during the growth period followed by 50% of the soil moisture depletion; (I2) water deficit stress from the start of the flowering stage (Z61) to the milking stage (Z77) associated with irrigation after 80% of the soil moisture depletion; (I3) water deficit stress from the beginning of the flowering stage (Z61) to the ripening stage (Z93) followed by irrigation after 80% of the soil moisture depletion; and (I4) water deficit stress from the start of the milking stage (Z77) to the ripening stage (Z93) followed by irrigation after 80% of the soil moisture depletion. The 3 cultivars of C1 (Sirvan), C2 (Pishtaz), and C3 (Marvdasht) were treated using the allocated sub-plots. The results revealed that by increasing the drought stress intensities on the wheat cultivars, the numbers of grains per spike and spikes per unit area, Thousand-Grain Weight (TGW), Grain Yield (GY), Biological Yield (BY), Harvest Index (HI) were decreased. The results of this study indicated that the wheat cultivars of Islamabad Agricultural Research Center had higher GY (5129 kg.h-1) and HI (42.05%), values than those of the Agricultural Research Center of Islamic Azad University, Kermanshah branch.
Keywords
Grain yield, Harvest index, Water deficit, Wheat, Yield components.
References
Alqudah A.M., Samarah N.H., Mullen R.E. (2011). Alternative farming systems, biotechnology, drought stress and ecological fertilization, drought stress effect on crop pollination, seed set, yield and quality. Sustainable Agriculture Rev 6: 193-213. https://doi.org/10.1016/j.cropro.2017.10.008
Bahrani A., Heidari-Sharifabad H., Tahmasebi-Sarvestani Z., Moafporian G. H., Ayenehband A. (2009.) Wheat response to nitrogen and postanthesis water deficit. The International Conference on CBEE 2009 pp: 33-34.
Bilal M., Rana R.M., Rehman S.U., Iqbal F., Ahmed J., Abid M. A., Ahmed Z., Hayat A. (2015). Evaluation of wheat genotypes for drought tolerance. J Green Physiol Genet Genom 1:11-21. https://doi.org/10.1371/journal.pone.0230954
Dolferus R., Ji X., Richards R. A. (2011). Abiotic stress and control of grain number in cereals. Plant Sci 181: 331-341. https://doi.org/10.1016/j.plantsci.2011.05.015
Farooq M., Hussain M., Siddique K.H. (2014). Drought stress in wheat during flowering and grain-filling periods. Critical Rev Plant Sci 33: 331-349. https://doi.org/10.1080/07352689.2014.875291
Gonzalez A., Bermejo V., Gimeno B.S. (2010). Effect of different physiological traits on grain yield in barley grown under irrigated and terminal water deficit conditions. J Agricultural Sci 148: 319-328. https://doi.org/10.1017/S0021859610000031
Hussain M., Malik M.A., Farooq M., Ashraf M.Y., Cheema M.A. (2008). Improving drought tolerance by exogenous application of glycine betaine and salicylic acid in sunflower. J Agron Crop Sci 194: 193-199. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1439-037X.2008.00305.x
Inamullah Z.A., Swati A., Siraj-u-Din L. (1999). Evaluation of lines for drought tolerance in heat (Triticum aestivum L.). Sci Khyber 12: 39-48.
Khan A.S., Ul-Allah S., Sadique S. (2010). Genetic variability and correlation among seedling traits of Wheat (Triticum aestivum L.) under water stress. Int J Agric Biol 2: 247-250.
Khanzada S., Ala A., Yasin Ashraf M., Shirazi M. U., Alam S. M., Ansari R., Ali M., Mujataba S. M.,. Khan M.A. (2001). Effect of water stress on yield and yield components of different Guar (Cyamopsis tetragonaloba L.) genotypes. Pak J Biol Sci 4: 371-374.
Kilic H., Yagbasanlar T. (2010). The effect of drought stress on grain yield, yield components and some quality traits of durum wheat (Triticum turgidum L.) cultivars. Not Bot Horti Agrobot Cluj Napoca 38: 164-170. https://www.notulaebotanicae.ro/index.php/nbha/article/view/4274/4437
Liu H., Searle I.R., Mather D.E., Able A. J., Able J.A. (2015). Morphological, physiological and yield responses of durum wheat to pre-anthesis water-deficit stress are genotype-dependent. Crop Pasture Sci 66: 1024-1038. https://www.publish.csiro.au/cp/CP15013
Moussavi-Nik M., Mobasser H. R., Mehraban A. (2007). Effect of water stress and potassium chloride on biological and grain yield of different wheat cultivars. Wheat Production Stressed Environ 12: 655-658. https://link.springer.com/chapter/10.1007%2F1-4020-5497-1_79
Nouri-Ganbalani A., Nouri-Ganbalani G., Hassanpanah D. (2009). Effects of drought stress condition on the yield and yield components of advanced wheat genotypes in Ardabil, Iran. J Food Agric Environ 7: 228-234. https://pubag.nal.usda.gov/catalog/774941
Prasad P.V.V., Pisipati S.R., Momcilovic I., Ristic Z. (2011). Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J Agron Crop Sci 197: 430-441. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1439-037X.2011.00477.x
Samarah N.H., Alqudah A.M., Amayreh J.A., McAndrews G.M. (2009). The effect of late-terminal drought stress on yield components of four barley cultivars. J Agron Crop Sci 195: 427-441.
Sanjari Pireivatlou A., Yazdansepas A. (2010). Evaluation of wheat (Triticum aestivum L.) genotypes under pre-and post-anthesis drought stress conditions. J Agr Sci Tech 10: 109-121. https://jast.modares.ac.ir/article-23-1620-en.html
Seki M., Narusaka M., Abe H., Kasuga M., Yamaguchi-Shinozaki K., Carninci P., Hayashizaki Y., Shinozaki K. (2001). Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13: 61-72. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC102214/
Taheri S., Saba J., Shekari F., Abdullah T.L. (2011). Effects of drought stress condition on the yield of spring wheat (Triticum aestivum L.) lines. Afr J Biotechnol 10: 18339-18348. https://www.ajol.info/index.php/ajb/article/view/98608
Tuberosa R. (2012). Phenotyping for drought tolerance of crops in the genomics era. Front Physiol 3. https://www.frontiersin.org/articles/10.3389/fphys.2012.00347/full
Ugarte C., Calderini D.F., Slafer G.A. (2007). Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crops Res 100: 240-248.
Zarafshar M., Akbarinia M., Askari H., Hosseini S.M, Rahaie M., Struve D., Striker G.G. (2014). Morphological, physiological and biochemical responses to soil water deficit in seedlings of three populations of wild pear tree (Pyrus boisseriana L.). Biotechnol Agron Soc Environ 18: 353-366. https://popups.uliege.be/1780-4507/index.php?id=11315
Introduction
Among all the abiotic stresses (drought, coldness, high salinity, etc.), drought is the most severe limiting factor in plant growth and crop production (Seki, et al. 2001). Drought is the condition, in which the amount of available water in the environment does not meet the requirements of a plant due to its high transpiration rates (Tuberosa, 2012). Wheat is one of the most important crops in Iran and worldwide. In all the areas where it is grown in Iran, drought is a major limiting factor influencing its yield. Plant growth, GY, and quality are severely affected by drought, which causes it to undergo molecular, biochemical, physiological, and morphological changes (Zarafshar, et al. 2014). Wheat yield under drought stress suffers a serious moisture deficit throughout its growth period from seedling to full maturity (Bilal, et al. 2015). A declining pattern has been evidenced to occur to wheat morphological yield and such features as height, number of grains per spike, number of spikes per plant, and TGW under drought conditions (Kilic & Yagbasanlar, 2010). For cereal crops, the extent to which water deficit stress reduces GY mainly depends on the developmental stage (Dolferus, et al. 2011). Water deficit stress enhances plant transpiration after anthesis and reduces grain size by significantly affecting grain filling (Sanjari Pireivatlou & Yazdansepas, 2010). The stress experienced before or during the anthesis stage largely influences the expected number of grains (Liu, et al. 2015). Several studies have been conducted on the spring and winter wheat to evaluate the effects of water deficit stress on crop production. Yield is mostly lowered when drought stress occurs during the heading or flowering and soft dough stages. GY is a critical and direct parameter for measuring drought damage. It has been well established that drought during the flowering and grain-filling stages can severely reduce wheat GY (Farooq, et al. 2014). Shrivelled grains would have resulted from reduced rate and duration of grain filling induced by water deficit conditions after anthesis (Prasad, et al. 2011). It is incumbent to investigate water deficit stress in the pre and post-anthesis stages, during which the number of grains per unit area and GW as the two major components of GY have to be determined, respectively. A higher number of grains per unit area is yielded via the translation of higher floret fertility during pre-anthesis development (Gonzalez, et al. 2010), whereas GW depends on the grain-filling stage supported by post-anthesis conditions (Ugarte, et al. 2007).
Materials and Methods
This research was conducted in the Agricultural Research Center of Islamic Azad University, Kermanshah branch, Iran (47°8ʹE, 34°23ʹN; elevation 1351 m) and Islamabad Agricultural Research Center, Kermanshah, Iran (47°26ʹ E, 34°8ʹ N; elevation 1346 m) during 2016-17. The experiments were carried out in a split-plot format based on a randomized complete block design in 3 replications. The main plots were allocated to 4 different irrigation regimes: (I1) full irrigation during the growth period followed by 50% of the soil moisture depletion; (I2) water deficit stress from the start of the flowering stage)Z61(to the milking stage(Z77) associated with irrigation after 80% of the soil moisture depletion; (I3) water deficit stress from the beginning of the flowering stage(Z61) to the ripening stage(Z93) followed by irrigation after 80% of the soil moisture depletion; and (I4) water deficit stress from the start of the milking stage(Z77) to the ripening stage(Z93) followed by irrigation after 80% of the soil moisture depletion. The sub-plots were allocated to the treatments of the three cultivars of C1 (Sirvan), C2 (Pishtaz), and C3 (Marvdasht). Based on De-marton's climate classification method, the climate of the Agricultural Research Center of Islamic Azad University, Kermanshah is cold and semi-arid with a mean annual rainfall of 435 mm and mean annual temperature of 13.5°C. Also, the climate of Islamabad Agricultural Research Center is cold and temperate with a mean annual rainfall of 538 mm and mean annual temperature of 10.5°C. In the Agricultural Research Center of Islamic Azad University of Kermanshah, the silty clay soil texture with a pH value of 7.3 consisted of the Total Organic Matter (TOM) of 1.8%, Electrical Conductivity (EC) of 1.3 dsm-1, Total Nitrogen (TN) of 8.13, available phosphorus of 0.04 mg.kg-1, available potassium of 330 mg.kg-1, and zinc, iron, and manganese contents of 1 mg.kg-1, 5.3 mg.kg-1, and 3.3 mg.kg-1, respectively. The loamy clay soil texture examined in Islamabad Agricultural Research Center had a pH value of 7.5, TOM of 1.6%, EC of 1.1 ds m-1, TN of 9.15, available phosphorus of 0.04 mg.kg-1, available potassium of 220 mg.kg-1, and zinc, iron, and manganese contents of 1.2 mg.kg-1, 5 mg.kg-1, and 4.2 mg.kg-1, respectively. At the beginning of the season, the experimental area was prepared with Moldboard plow/Disking (MD). Each plot included 8 rows of 5 m length separated by a space of 20 cm, while the plots and blocks were kept apart within the distances of 1 m and 2 m, respectively. 400 seeds per square meter were considered for the wheat density. The planting procedure was arranged during the 2nd week of November. The 1st step of irrigation was immediately taken after seed planting. Water quantity for each irrigation cycle was determined based on the areas of the test plots and continuous measurements of their moisture contents using a wet HH2 device. Drought stress was imposed on the treatments by stopping irrigation during the targeted growth stages. Irrigation of all the plots through an installed pipeline system was performed by controlling the water input volume for each plot using an adjustable counter. Hand weeding was carried out to keep the crops free from weeds throughout the growth period. At the physiological maturity stage, the spikes existing in the square meter of two middle rows were counted. 10 random samples of the grains harvested in each plot were selected to determine TGW. 10 spikes from each plot were randomly sampled, sun-dried, and threshed for determining the number of grains per spike. The GY, BY, and HI was measured by harvesting the plants of the 4th and 5th rows of 3 m length from each plot centre during the maturity stage.
The two software systems of MSTATC and SPSS were utilized to conduct the obtained data analysis. The targeted traits underwent the analysis of variance. Duncan's Multiple Range Test (MRT) was employed for comparing the means (p=0.05) and Pearson’s correlation analysis was applied for assessing the correlations between the parameters. Finally, Microsoft Excel was used for constructing the diagrams.
Results and Discussion
Plant Height (PH)
Water deficit stress caused impaired mitosis and cell elongation and expansion and resulted in lowered growth rates and yield traits (Hussain, et al. 2008). As displayed in Tab.1, cultivar and water deficit stress have significant impacts on the PH levels (p<0.01). The highest and lowest PH values were seen to be related to Sirvan (C1) and Marvdasht (C3) cultivars, respectively (Tab. 1). Correspondingly, the highest and lowest PH values were found to belong to Treatments I1 and I3, respectively. Khanzada, et al. (2001) reported PH to be significantly decreased under drought stress. Similarly, Inamullah, et al. (1999) stated that Shoot Height (SH) in wheat cultivars can undergo a significant reduction under water stress conditions when compared to the irrigated plants.
| Treatments | PH (cm) | SPSM | GPS | TGW (g) | GY (kg.h-1) | BY (kg.h-1) | HI (%) | |
|---|---|---|---|---|---|---|---|---|
| Water deficit stress | ||||||||
| I1 | 90.42a | 710.2b | 30.24a | 42.32a | 6935a | 14952a | 49.83a | |
| I2 | 71.13b | 709.2b | 22.15c | 34.89b | 4359c | 12671b | 35.80c | |
| I3 | 59.32c | 704.4b | 18.42c | 33.34b | 3590d | 11720c | 30.98d | |
| I4 | 86.34a | 720.7a | 26.44b | 36.18b | 5356b | 11920b | 42.83b | |
| Cultivar | ||||||||
| C1 | 87.92a | 719.3a | 26.77a | 39.59a | 5954a | 13943a | 45.43a | |
| C2 | 75.89b | 717.3a | 24.29b | 36.66b | 5873a | 12645b | 41.62b | |
| C3 | 72.22b | 708.2b | 22.58c | 32.50c | 5429b | 11993b | 36.31c | |
| Location | ||||||||
| Azad | 73.72b | 713.3a | 25.23a | 34.71b | 4751b | 11865a | 39.92b | |
| Islamabad | 80.45a | 717.0a | 24.28a | 37.00a | 5129a | 12963a | 42.05a | |
| Water deficit stress | ** | ** | ** | ** | ** | ** | ** | |
| Cultivar | ** | ** | ** | ** | ** | * | ** | |
| Water deficit stress × Cultivar | ns | ** | ** | ** | ns | ns | ** | |
| Location | * | ns | ** | ns | * | ns | ** | |
| CV% | 5.62 | 12.34 | 7.84 | 11.17 | 4.84 | 11.63 | 18.28 | |
| PH: Plant Height, SPSM: Spike Per Square Meter, GPS: Grains Per Spike, TGW: Thousand-Grain Weight, GY: Grain Yield, BY: Biological Yield, HI: Harvest Index. Within treatment means followed by the same letter are not significantly at p<0.05 according to Duncan’s multiple range test. *: P<0.05, **: p<0.01, ns: Non signification. | ||||||||
Table 1. Effect of cultivar and irrigation on agronomical characteristics in wheat (data derived mean two location).
Number of spikes per square meter
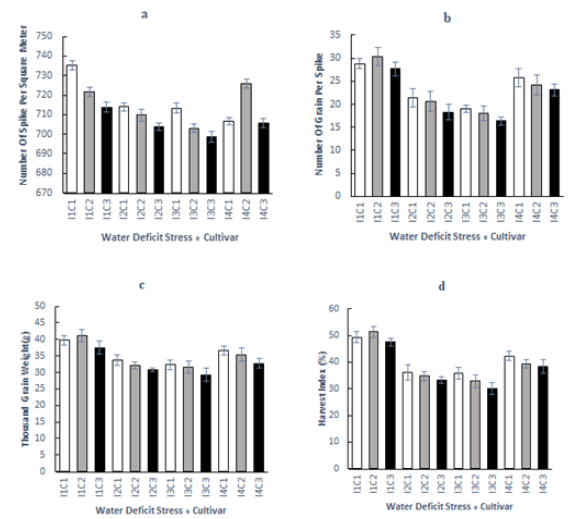
As shown in Tab.1, water deficit stress and cultivar have significant effects on the number of spikes per square meter (p<0.01). Also, the interaction between cultivar and water deficit stress is significant (p<0.01). In this experiment, the highest decrease in the number of spikes per square meter belonged to Treatment I3 as compared to the control treatment (I1) (Tab. 1). This study showed that Sirvan cultivar (C1) had a higher number of spikes per square meter than those of Marvdasht (C3) and Pishtaz (C2) cultivars. Sirvan (C1) and Marvdasht (C3) cultivars respectively displayed the highest and lowest numbers of spikes per square meter under mild (I4), moderate (I2), and severe (I3) water-deficit stress conditions as represented in Fig. 1a. This result is in good agreement with those of other studies, which have shown that water deficit stress has a significant negative correlation with the number of spikes per square meter (Samarah, et al. 2009; Khan, et al. 2010).
Figure 1: Effect of cultivar and water deficit stress on a: Number of spike per square meter; b: Number of grain per spike; c: Thousand-grain weight; d: Harvest index.
Number of grains per spike
From the statistical viewpoint, significant results were obtained based on the analysis of variance representing changes in the number of grains per spike under the impacts of water deficit stress and cultivar (p<0.01). In addition, the interaction between cultivar and water deficit stress affecting the number of grains per spike was significant (p<0.01), the results of which are illustrated in Tab. 1. The highest and lowest numbers of grains per spike were found in the treatments of I1C2 and I3C3, respectively (Fig. 1b). Similar results were obtained by Taheri, et al. (2011) when working on different wheat cultivars.
TGW
Water deficit stress and cultivar had significant effects on TGW values (p<0.01). The interaction between cultivar and water deficit stress had a significant impact on TGW (p<0.01) (Tab. 1). In this experiment, the highest decrease in the number of spikes per square meter belonged to Treatment I3 as compared to the control treatment (I1) (Tab. 1). Sirvan (C1) and Marvdasht (C3) cultivars exhibited the highest and lowest TGW values, respectively. As shown in Fig. 1c, the highest and lowest TGW values appear in the treatments of I1C2 and I3C3, respectively. Raynolds, et al. (2000) declared that water deficit stress during the post-anthesis phase lowered grain-filling rates and subsequently TGW values. Taheri, et al. (2011) stated that water deficit stress during the post-anthesis stage could disrupt the processes of photosynthesis and stored substance transfer to the grains and consequently decrease TGW. This finding is consistent with those achieved in our experiments.
GY, BY, and HI
As depicted in Tab. 1, water deficit stress and cultivar have significant effects on GY, BY, and HI (p<0.01). Moreover, the interaction between cultivar and water deficit stress demonstrates a significant impact (p<0.01) on HI. The highest and lowest GY, BY, and HI values were observed to be related to Sirvan (C1) and Marvdasht (C3) cultivars, respectively (Tab.1). Additionally, the highest and lowest GY, BY, and HI values were found to belong to the treatments of I1 and I3, respectively. Also, the highest and lowest HI values were seen to be related to the treatments of I1C2 and I3C3, respectively (Fig. 1d). As reported by Samarah, et al. (2009) and Alqudah, et al. (2011), GY could be drastically alleviated by water-deficit stress during the grain-filling period. Bahrani, et al. (2009) declared declined GY of wheat to be caused by water deficit stress at the post-anthesis phase. Similar results were obtained by Moussavi-Nik, et al. (2007), who discovered decreased wheat GY as a result of no irrigation treatment after pollination. All these findings are congruent with those of our experiments. In this regard, Nouri-Ganbalani, et al. (2009) came to the same conclusions.
Agronomical traits
The results of the analysis of variance revealed significant differences between the PHs and GYs, (p=0.05) of the different cultivars of the two studied regions, while highly significant differences (p=0.01) were evidenced between their numbers of grains per spike and HIs (Tab. 1). GYand HI were higher in all the experimental treatments dealt with in Islamabad Agricultural Research Center compared to the Agricultural Research Center of Islamic Azad University of Kermanshah (Tab. 1). This was due to the higher distribution patterns of precipitation during the winter and spring in Islamabad region in contrast to the higher mean temperatures during March and April in the region of Islamic Azad University of Kermanshah in the year of the experiment. Moreover, the mean temperatures of the latter region were higher during the terminated wheat growth periods of May and June, thus resulting in the shortened reproductive period of wheat and its reduced yield (Tab. 2).
| Agricultural Research Center of Islamic Azad University, Kermanshah Branch | Agricultural Research Center of Islamabad | |||
|---|---|---|---|---|
| Air temperature °C | Precipitation mm | Air temperature °C | Precipitation mm | |
| Months | Monthly Mean | Monthly Mean | Monthly Mean | Monthly Mean |
| 10 | 18.7 | 0 | 16.9 | 0 |
| 11 | 10.6 | 115.9 | 8.7 | 131 |
| 12 | 3.1 | 0.5 | 2.9 | 0.8 |
| 1 | 4.4 | 4.9 | 2.2 | 10.4 |
| 2 | 3 | 76.7 | 1.5 | 68.2 |
| 3 | 4.4 | 23.3 | 2.6 | 34.3 |
| 4 | 11.8 | 24.4 | 13.3 | 45.7 |
| 5 | 18.4 | 15.6 | 17 | 17.9 |
| 6 | 24.9 | 0 | 22.5 | 0 |
| 7 | 28.1 | 0 | 27.5 | 0 |
| 8 | 29.8 | 0 | 27.4 | 0 |
| 9 | 25.9 | 0 | 23.2 | 0 |
Table 2. Meteorological condition in 2016-2017.
Conclusions
The results of this investigation revealed reduced wheat yield and some of its components in all the studied cultivars under water deficit stress conditions. The different abilities of drought tolerance in the wheat cultivars could be reflected by their differential responses to the varied imposed water deficit stresses. On the whole, our findings and the results achieved by others suggested the necessity of taking the strategies of a high number of grains per spike and GW for enhancing wheat yields under water deficit stress conditions. Nonetheless, high stability and potential for yield were documented for ‘Sirvan’ cultivar under drought stress conditions. Hence, these relationships can serve as the selection criteria for screening the cultivars of potentially high drought resistance and GYs when having to deal with water-deficit stress conditions.
Acknowledgement
This work was supported by a grant from the Research Council of Islamic Azad University, Kermanshah Branch, Iran.
Copyright: ©The Author(s) 2022. Published by Andriy Novikov, State Natural History Museum NAS of Ukraine on behalf of Modern Phytomorphology. This is an open access article under the Creative Commons BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/) freely available on https://phytomorphology.org/. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.